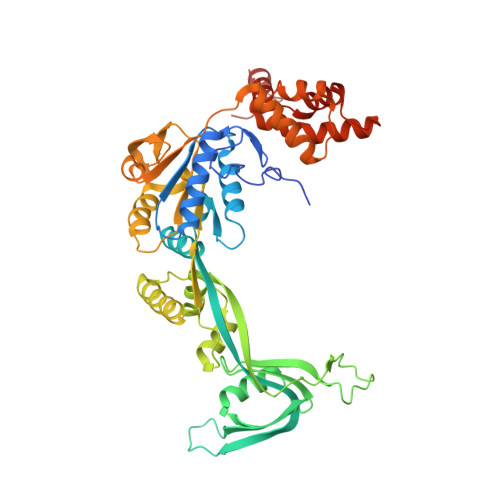
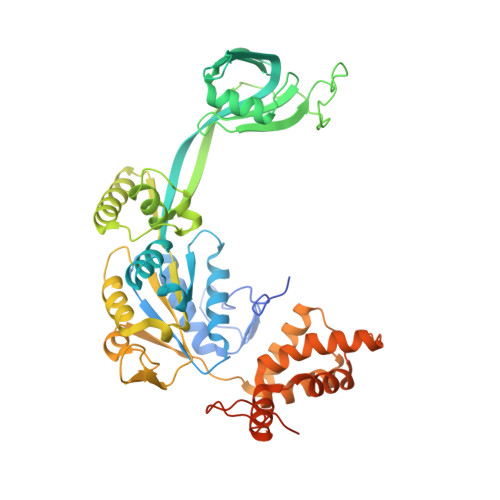
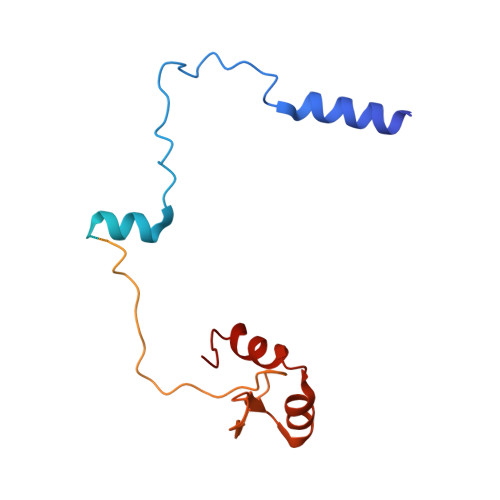
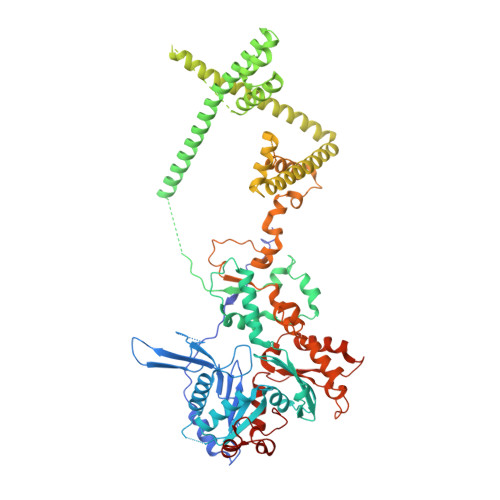
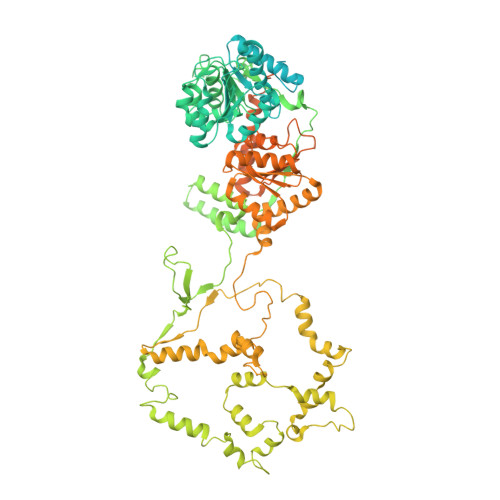Hexasome-INO80 complex reveals structural basis of noncanonical nucleosome remodeling.
Zhang, M., Jungblut, A., Kunert, F., Hauptmann, L., Hoffmann, T., Kolesnikova, O., Metzner, F., Moldt, M., Weis, F., DiMaio, F., Hopfner, K.P., Eustermann, S.(2023) Science 381: 313-319
- PubMed: 37384673
- DOI: https://doi.org/10.1126/science.adf6287
- Primary Citation of Related Structures:
8OO7, 8OO9, 8OOA, 8OOC, 8OOF, 8OOK, 8OOP, 8OOR, 8OOS, 8OOT - PubMed Abstract:
Loss of H2A-H2B histone dimers is a hallmark of actively transcribed genes, but how the cellular machinery functions in the context of noncanonical nucleosomal particles remains largely elusive. In this work, we report the structural mechanism for adenosine 5'-triphosphate-dependent chromatin remodeling of hexasomes by the INO80 complex. We show how INO80 recognizes noncanonical DNA and histone features of hexasomes that emerge from the loss of H2A-H2B. A large structural rearrangement switches the catalytic core of INO80 into a distinct, spin-rotated mode of remodeling while its nuclear actin module remains tethered to long stretches of unwrapped linker DNA. Direct sensing of an exposed H3-H4 histone interface activates INO80, independently of the H2A-H2B acidic patch. Our findings reveal how the loss of H2A-H2B grants remodelers access to a different, yet unexplored layer of energy-driven chromatin regulation.
Organizational Affiliation:
Structural and Computational Biology Unit, European Molecular Biology Laboratory (EMBL), Heidelberg, Germany.