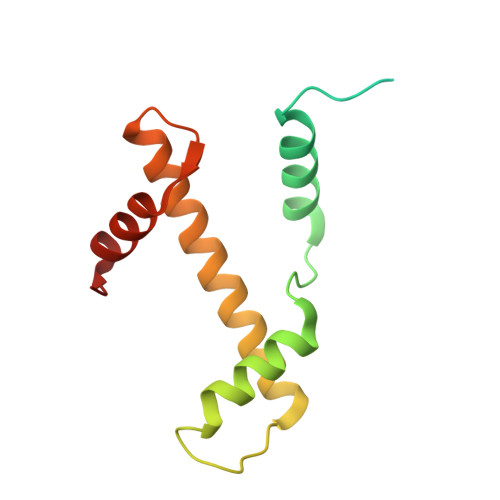
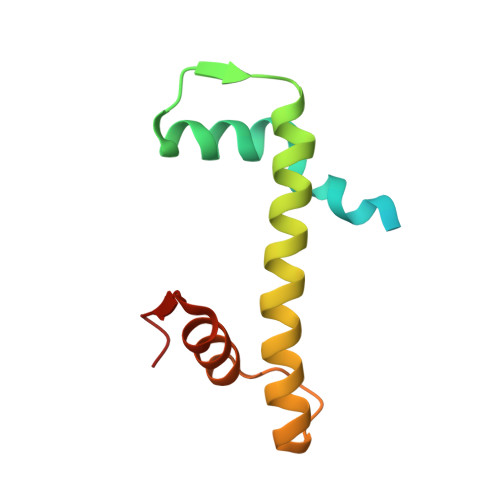
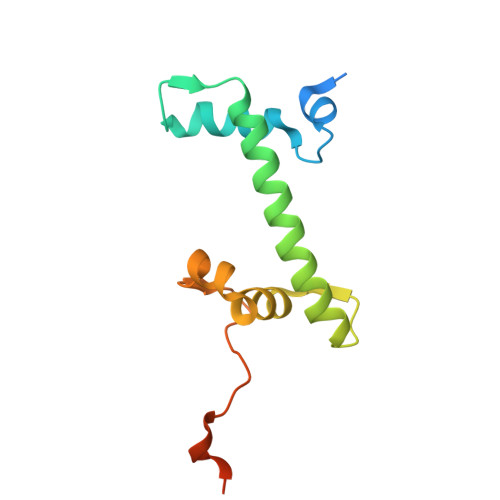
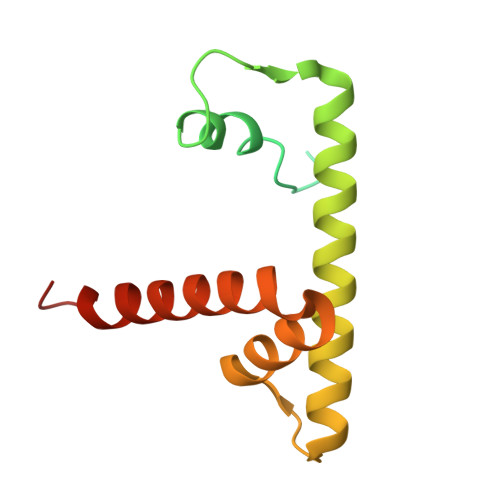
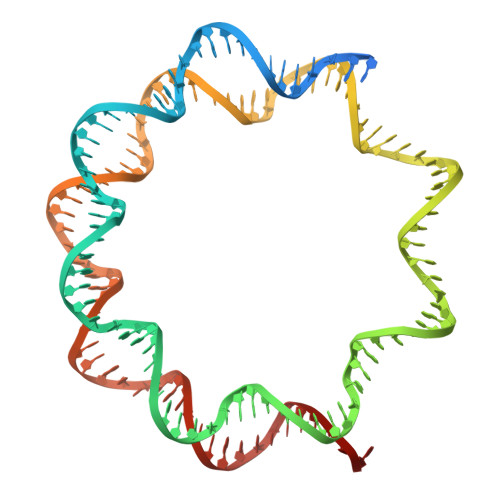
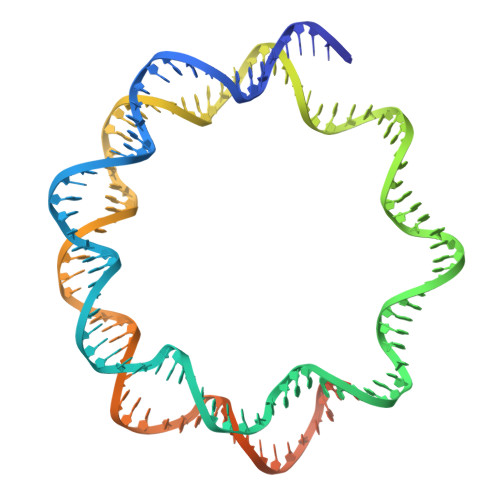
H2B.W2, a spermatocyte-specific histone variant, disrupts nucleosome stability, and reduces chromatin compaction.
Nguyen, A.T.T., Ding, D., Bai, X., Pang, M.Y.H., Deng, M., Liu, Y., Jin, T., Xu, Z., Zhang, Y., Zhai, Y., Yan, Y., Ishibashi, T.(2025) Nucleic Acids Res 53
- PubMed: 40874595
- DOI: https://doi.org/10.1093/nar/gkaf825
- Primary Citation of Related Structures:
9JC6 - PubMed Abstract:
Spermatogenesis is a highly regulated process that requires precise chromatin remodeling, which includes the incorporation of testis-specific histone variants. While several of these variants have been characterized, the role of H2B.W2, a member of the H2BW family, remains largely unclear. Here, we showed that H2B.W2 expression occurs mainly in spermatocytes, slightly later than its paralog H2B.W1. Cryo-electron microscopy analysis of H2B.W2-containing nucleosomes reveals a more relaxed conformation compared to canonical nucleosomes caused by weakened interactions between the outer DNA turn and the histone core. We pinpointed the N-terminal tail and α2 helix of H2B.W2 as critical regions for nucleosome destabilization. Furthermore, we identify G73 within the L1 loop as a key residue involved in disrupting higher-order chromatin structure. Our findings suggest that H2B.W2-mediated nucleosome and chromatin destabilization may play a role in regulating gene expression during spermatogenesis, with potential implications for sperm development and function.
- Division of Life Science, The Hong Kong University of Science and Technology, Clear Water Bay, NT, HKSAR, China.
Organizational Affiliation: