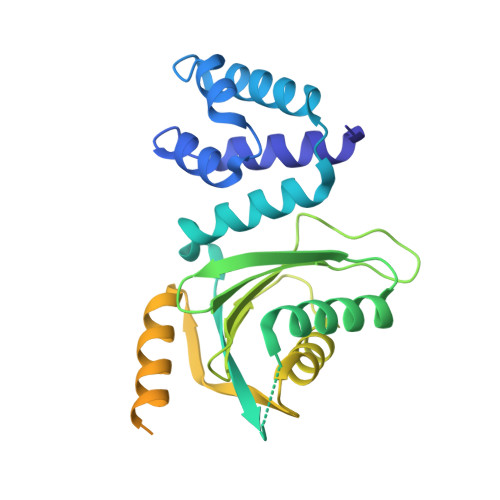
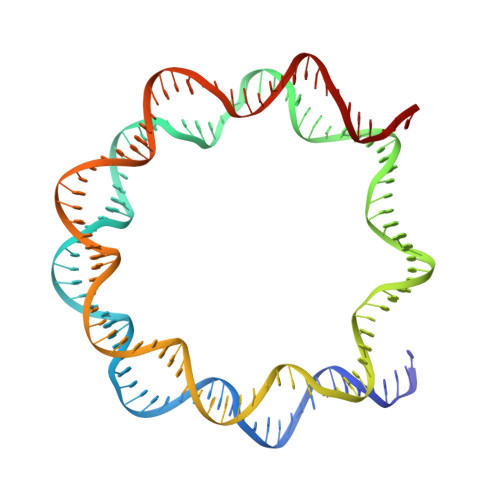
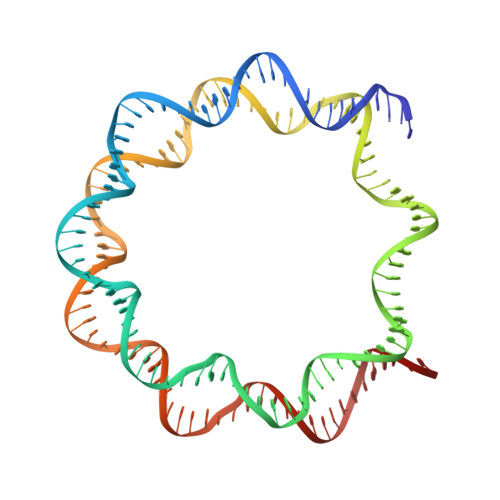
CENP-N promotes the compaction of centromeric chromatin.
Zhou, K., Gebala, M., Woods, D., Sundararajan, K., Edwards, G., Krzizike, D., Wereszczynski, J., Straight, A.F., Luger, K.(2022) Nat Struct Mol Biol 29: 403-413
- PubMed: 35422519
- DOI: https://doi.org/10.1038/s41594-022-00758-y
- Primary Citation of Related Structures:
7U46, 7U47, 7U4D - PubMed Abstract:
The histone variant CENP-A is the epigenetic determinant for the centromere, where it is interspersed with canonical H3 to form a specialized chromatin structure that nucleates the kinetochore. How nucleosomes at the centromere arrange into higher order structures is unknown. Here we demonstrate that the human CENP-A-interacting protein CENP-N promotes the stacking of CENP-A-containing mononucleosomes and nucleosomal arrays through a previously undefined interaction between the α6 helix of CENP-N with the DNA of a neighboring nucleosome. We describe the cryo-EM structures and biophysical characterization of such CENP-N-mediated nucleosome stacks and nucleosomal arrays and demonstrate that this interaction is responsible for the formation of densely packed chromatin at the centromere in the cell. Our results provide first evidence that CENP-A, together with CENP-N, promotes specific chromatin higher order structure at the centromere.
Organizational Affiliation:
Department of Biochemistry, University of Colorado at Boulder, Boulder, CO, USA.