Contribution of histone N-terminal tails to the structure and stability of nucleosomes
Iwasaki, W., Miya, Y., Horikoshi, N., Osakabe, A., Taguchi, H., Tachiwana, H., Shibata, T., Kagawa, W., Kurumizaka, H.(2013) FEBS Open Bio 3: 363-369
- PubMed: 24251097
- DOI: https://doi.org/10.1016/j.fob.2013.08.007
- Primary Citation of Related Structures:
3W96, 3W97, 3W98, 3W99 - PubMed Abstract:
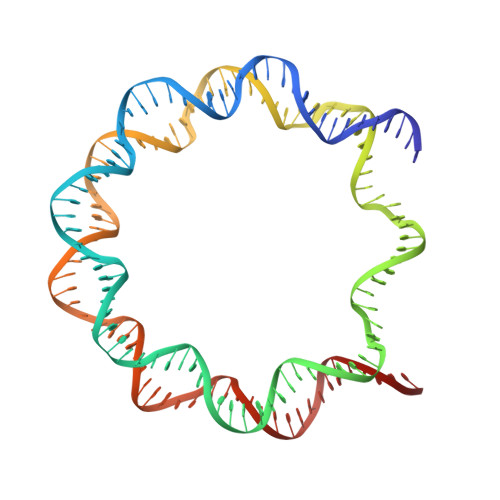
Histones are the protein components of the nucleosome, which forms the basic architecture of eukaryotic chromatin. Histones H2A, H2B, H3, and H4 are composed of two common regions, the "histone fold" and the "histone tail". Many efforts have been focused on the mechanisms by which the post-translational modifications of histone tails regulate the higher-order chromatin architecture. On the other hand, previous biochemical studies have suggested that histone tails also affect the structure and stability of the nucleosome core particle itself. However, the precise contributions of each histone tail are unclear. In the present study, we determined the crystal structures of four mutant nucleosomes, in which one of the four histones, H2A, H2B, H3, or H4, lacked the N-terminal tail. We found that the deletion of the H2B or H3 N-terminal tail affected histone-DNA interactions and substantially decreased nucleosome stability. These findings provide important information for understanding the complex roles of histone tails in regulating chromatin structure.
Organizational Affiliation:
Laboratory of Structural Biology, Graduate School of Advanced Science and Engineering, Waseda University, 2-2 Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan ; RIKEN, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan.