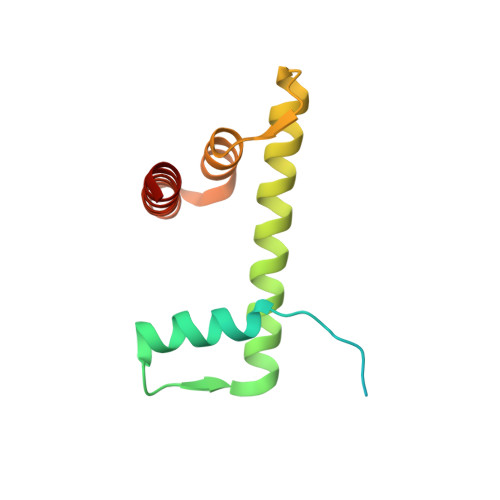
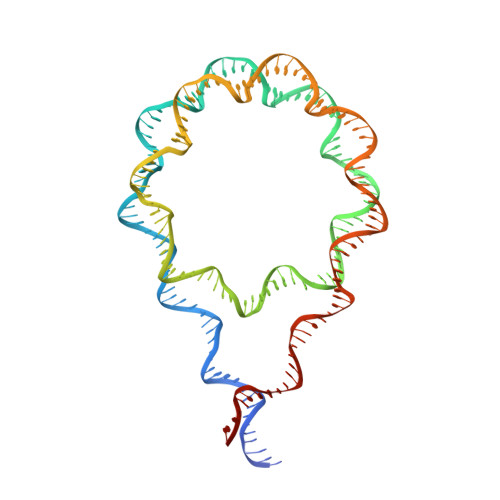
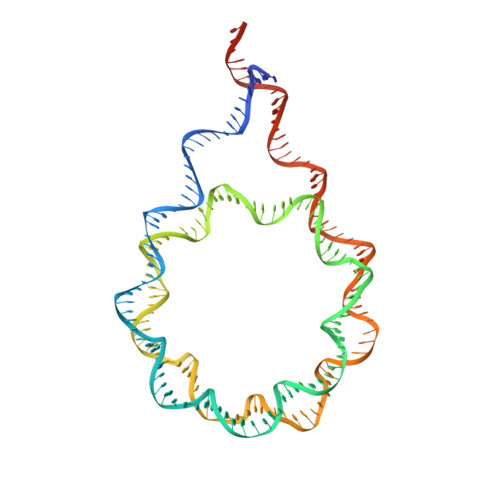
Nucleosome dyad determines the H1 C-terminus collapse on distinct DNA arms.
Louro, J.A., Boopathi, R., Beinsteiner, B., Mohideen Patel, A.K., Cheng, T.C., Angelov, D., Hamiche, A., Bendar, J., Kale, S., Klaholz, B.P., Dimitrov, S.(2023) Structure 31: 201
- PubMed: 36610392
- DOI: https://doi.org/10.1016/j.str.2022.12.005
- Primary Citation of Related Structures:
8AAG - PubMed Abstract:
Nucleosomes are symmetric structures. However, binding of linker histones generates an inherently asymmetric H1-nucleosome complex, and whether this asymmetry is transmitted to the overall nucleosome structure, and therefore also to chromatin, is unclear. Efforts to investigate potential asymmetry due to H1s have been hampered by the DNA sequence, which naturally differs in each gyre. To overcome this issue, we designed and analyzed by cryo-EM a nucleosome reconstituted with a palindromic (601L) 197-bp DNA. As in the non-palindromic 601 sequence, H1 restricts linker DNA flexibility but reveals partial asymmetrical unwrapping. However, in contrast to the non-palindromic nucleosome, in the palindromic nucleosome H1 CTD collapses to the proximal linker. Molecular dynamics simulations show that this could be dictated by a slightly tilted orientation of the globular domain (GD) of H1, which could be linked to the DNA sequence of the nucleosome dyad.
- Centre for Integrative Biology (CBI), Department of Integrated Structural Biology, IGBMC (Institute of Genetics and of Molecular and Cellular Biology), 1 rue Laurent Fries, 67404 Illkirch, France.
Organizational Affiliation: