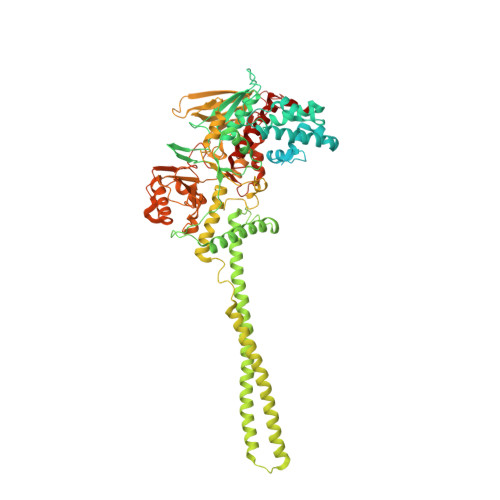
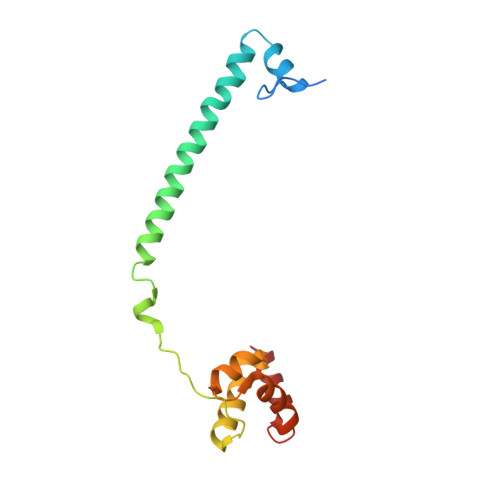
Covalent adduct Grob fragmentation underlies LSD1 demethylase-specific inhibitor mechanism of action and resistance.
Waterbury, A.L., Caroli, J., Zhang, O., Tuttle, P.R., Liu, C., Li, J., Park, J.S., Hoenig, S.M., Barone, M., Furui, A., Mattevi, A., Liau, B.B.(2025) Nat Commun 16: 3156-3156
- PubMed: 40175327
- DOI: https://doi.org/10.1038/s41467-025-57477-3
- Primary Citation of Related Structures:
8BOP, 8BOX, 8F2Z, 8F30, 8F59, 8F6S, 8FDV, 8FJ4, 8FJ7, 8FQJ, 8FRI, 8FRQ, 8FRV, 8FSK, 8GJ6, 8UL6, 8UL8, 8ULB, 8ULC, 8UMQ, 8UNI, 8UOM, 9EL7, 9EL8, 9ELA - PubMed Abstract:
Chromatin modifiers often work in concert with transcription factors (TFs) and other complex members, where they can serve both enzymatic and scaffolding functions. Due to this, active site inhibitors targeting chromatin modifiers may perturb both enzymatic and nonenzymatic functions. For instance, the antiproliferative effects of active-site inhibitors targeting lysine-specific histone demethylase 1A (LSD1) are driven by disruption of a protein-protein interaction with growth factor independence 1B (GFI1B) rather than inhibition of demethylase activity. Recently, next-generation precision LSD1 covalent inhibitors have been developed, which selectively block LSD1 enzyme activity by forming a compact N-formyl flavin adenine dinucleotide (FAD) adduct that spares the GFI1B interaction. However, the mechanism accounting for N-formyl-FAD formation remains unclear. Here we clarify the mechanism of these demethylase-specific inhibitors of LSD1, demonstrating that the covalent inhibitor-FAD adduct undergoes a Grob fragmentation. Using inhibitor analogs and structural biology, we identify structure-activity relationships that promote this transformation. Furthermore, we unveil an unusual drug resistance mechanism whereby distal active-site mutations can promote inhibitor-adduct Grob fragmentation even for previous generation compounds. Our study uncovers the unique Grob fragmentation underlying the mechanism of action of precision LSD1 enzyme inhibitors, offering insight into their reactivity with broader implications for drug resistance.
- Department of Chemistry and Chemical Biology, Cambridge, MA, 02138, USA.
Organizational Affiliation: