Distal drug resistance mutations promote covalent inhibitor-adduct Grob fragmentation in LSD1
Caroli, J., Mattevi, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lysine-specific histone demethylase 1A | 871 | Homo sapiens | Mutation(s): 0 Gene Names: KDM1A, AOF2, KDM1, KIAA0601, LSD1 EC: 1.14.99.66 | 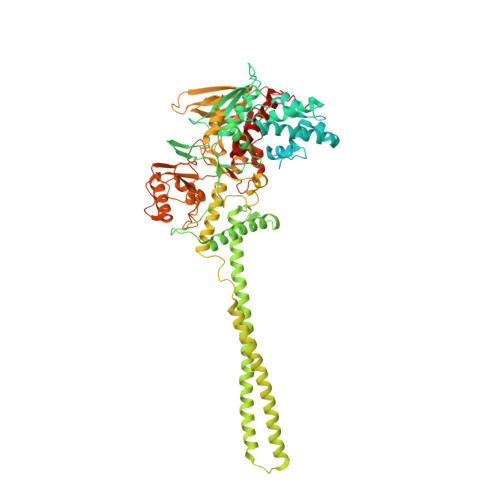 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O60341 (Homo sapiens) Explore O60341 Go to UniProtKB: O60341 | |||||
PHAROS: O60341 GTEx: ENSG00000004487 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O60341 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| REST corepressor 1 | 144 | Homo sapiens | Mutation(s): 0 Gene Names: RCOR1, KIAA0071, RCOR | 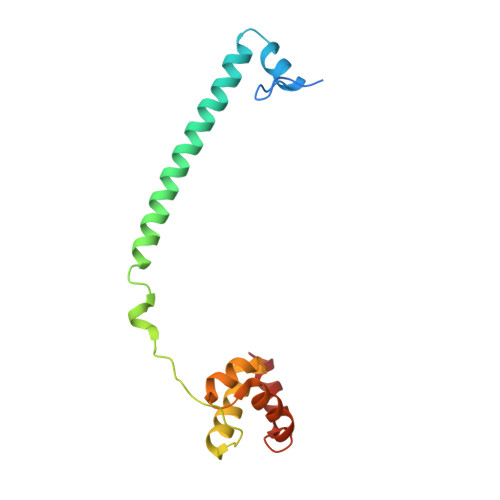 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9UKL0 (Homo sapiens) Explore Q9UKL0 Go to UniProtKB: Q9UKL0 | |||||
PHAROS: Q9UKL0 GTEx: ENSG00000089902 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9UKL0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Zinc finger protein SNAI1 | 9 | Homo sapiens | Mutation(s): 0 Gene Names: SNAI1, SNAH | 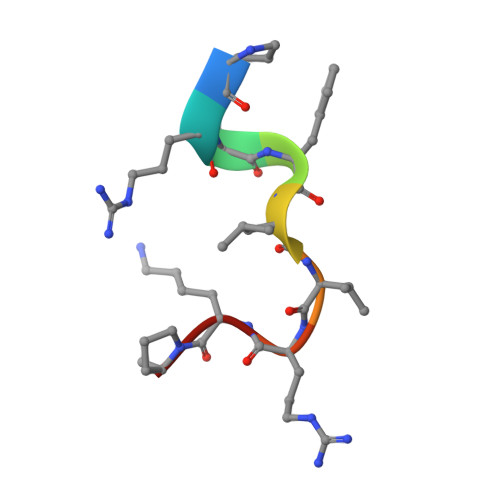 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O95863 (Homo sapiens) Explore O95863 Go to UniProtKB: O95863 | |||||
PHAROS: O95863 GTEx: ENSG00000124216 | |||||
Entity Groups | |||||
| UniProt Group | O95863 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HUF (Subject of Investigation/LOI) Query on HUF | D [auth A] | [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-bis(oxidanyl)oxolan-2-yl]methoxy-oxidanyl-phosphoryl] [(2R,3S,4S)-5-[5-methanoyl-7,8-dimethyl-2,4-bis(oxidanylidene)-1H-benzo[g]pteridin-10-yl]-2,3,4-tris(oxidanyl)pentyl] hydrogen phosphate C28 H35 N9 O16 P2 KZQASMOVHMAAKH-MZWSMYJRSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 119.298 | α = 90 |
| b = 180.388 | β = 90 |
| c = 232.884 | γ = 90 |
| Software Name | Purpose |
|---|---|
| Aimless | data scaling |
| PHENIX | refinement |
| XDS | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Italian Association for Cancer Research | Italy | IG19808 |