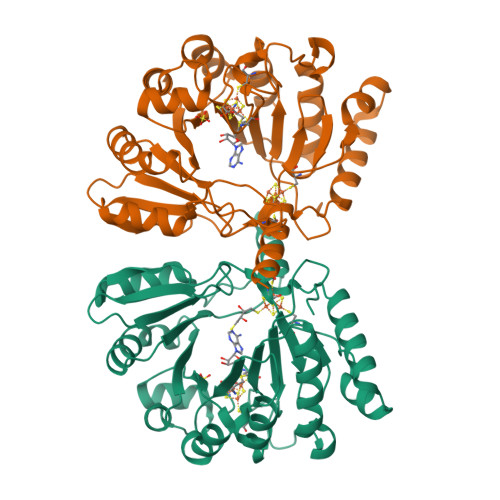
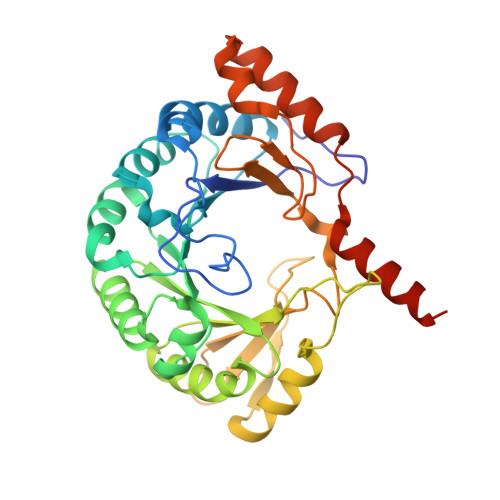
Crystal structure of the S-adenosylmethionine-dependent enzyme MoaA and its implications for molybdenum cofactor deficiency in humans.
Hanzelmann, P., Schindelin, H.(2004) Proc Natl Acad Sci U S A 101: 12870-12875
- PubMed: 15317939
- DOI: https://doi.org/10.1073/pnas.0404624101
- Primary Citation of Related Structures:
1TV7, 1TV8 - PubMed Abstract:
The MoaA and MoaC proteins catalyze the first step during molybdenum cofactor biosynthesis, the conversion of a guanosine derivative to precursor Z. MoaA belongs to the S-adenosylmethionine (SAM)-dependent radical enzyme superfamily, members of which catalyze the formation of protein and/or substrate radicals by reductive cleavage of SAM by a [4Fe-4S] cluster. A defined in vitro system is described, which generates precursor Z and led to the identification of 5'-GTP as the substrate. The structures of MoaA in the apo-state (2.8 angstroms) and in complex with SAM (2.2 angstroms) provide valuable insights into its mechanism and help to define the defects caused by mutations in the human ortholog of MoaA that lead to molybdenum cofactor deficiency, a usually fatal disease accompanied by severe neurological symptoms. The central core of each subunit of the MoaA dimer is an incomplete triosephosphate isomerase barrel formed by the N-terminal part of the protein, which contains the [4Fe-4S] cluster typical for SAM-dependent radical enzymes. SAM is the fourth ligand to the cluster and binds to its unique Fe as an N/O chelate. The lateral opening of the incomplete triosephosphate isomerase barrel is covered by the C-terminal part of the protein containing an additional [4Fe-4S] cluster, which is unique to MoaA proteins. Both FeS clusters are separated by approximately 17 angstroms, with a large active site pocket between. The noncysteinyl-ligated unique Fe site of the C-terminal [4Fe-4S] cluster is proposed to be involved in the binding and activation of 5'-GTP.
Organizational Affiliation:
Department of Biochemistry, Center for Structural Biology, State University of New York, Stony Brook, NY 11794-5115, USA.