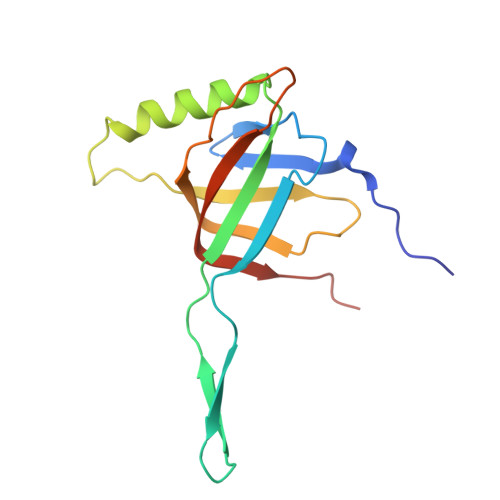
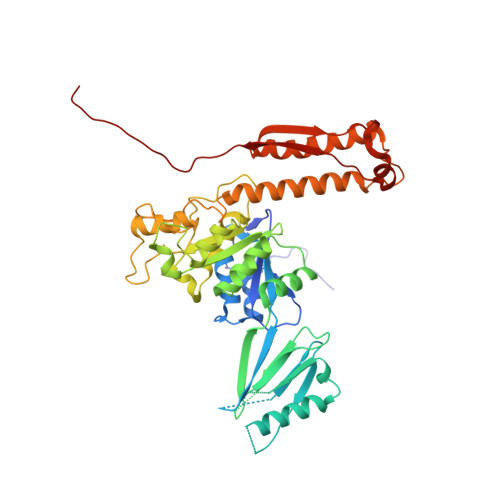
Cryo-EM structure of bacteriophage Bas63 reveals structural conservation and diversity in the Felixounavirus genus.
Hodgkinson-Bean, J., Ayala, R., McJarrow-Keller, K., Cassin, L., Rutter, G.L., Crowe, A.J.M., Wolf, M., Bostina, M.(2025) Sci Adv 11: eadx0790-eadx0790
- PubMed: 41223280
- DOI: https://doi.org/10.1126/sciadv.adx0790
- Primary Citation of Related Structures:
9MT4, 9MT5 - PubMed Abstract:
The BASEL phage collection was developed to provide access to diverse bacteriophages, distinct from model phages. Escherichia phage JohannRWettstein (Bas63), a myophage in the collection, is a member of the subfamily Ounavirinae and the Felixounavirus genus. Using cryo-electron microscopy, we investigated Bas63's structure to explore its evolutionary relationships and functional adaptations. Our structures reveal a series of gene products: (i) a capsid decorated with β-tulip proteins at three-fold symmetry axes and a Hoc-like protein at hexamer centers, (ii) a conserved connector with an additional 12-fold ring of collar proteins that extend unique whisker proteins that are structurally related to podophage GP4 tail fibers, and (iii) a baseplate with long tail fibers resembling a contracted form of T4's long tail fibers. Sequence conservation analysis of Bas63 structural proteins across ICTV-recognized Felixounavirus' supports its role as a structural model for Felixounavirus evolution. This study advances the mechanistic understanding of phage architecture and reinforces the structural mosaicism of bacteriophages.
- Department of Microbiology and Immunology, University of Otago, Dunedin, New Zealand.
Organizational Affiliation: