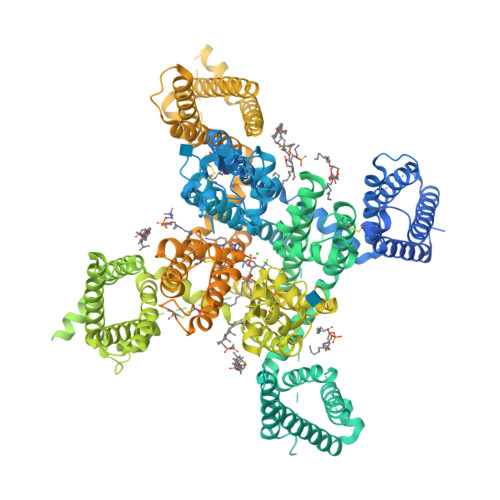
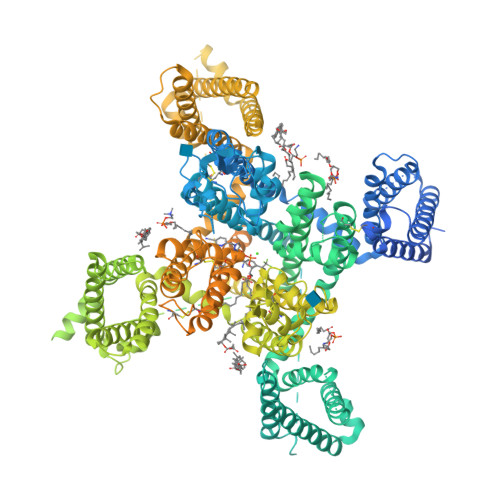
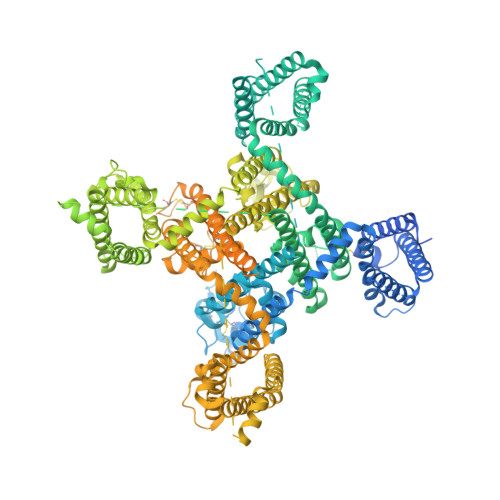
Structural basis for human Ca v 3.2 inhibition by selective antagonists.
Huang, J., Fan, X., Jin, X., Lyu, C., Guo, Q., Liu, T., Chen, J., Davakan, A., Lory, P., Yan, N.(2024) Cell Res 34: 440-450
- PubMed: 38605177
- DOI: https://doi.org/10.1038/s41422-024-00959-8
- Primary Citation of Related Structures:
9AYG, 9AYH, 9AYJ, 9AYK, 9AYL - PubMed Abstract:
The Ca v 3.2 subtype of T-type calcium channels has been targeted for developing analgesics and anti-epileptics for its role in pain and epilepsy. Here we present the cryo-EM structures of Ca v 3.2 alone and in complex with four T-type calcium channel selective antagonists with overall resolutions ranging from 2.8 Å to 3.2 Å. The four compounds display two binding poses. ACT-709478 and TTA-A2 both place their cyclopropylphenyl-containing ends in the central cavity to directly obstruct ion flow, meanwhile extending their polar tails into the IV-I fenestration. TTA-P2 and ML218 project their 3,5-dichlorobenzamide groups into the II-III fenestration and place their hydrophobic tails in the cavity to impede ion permeation. The fenestration-penetrating mode immediately affords an explanation for the state-dependent activities of these antagonists. Structure-guided mutational analysis identifies several key residues that determine the T-type preference of these drugs. The structures also suggest the role of an endogenous lipid in stabilizing drug binding in the central cavity.
Organizational Affiliation:
Department of Molecular Biology, Princeton University, Princeton, NJ, USA.