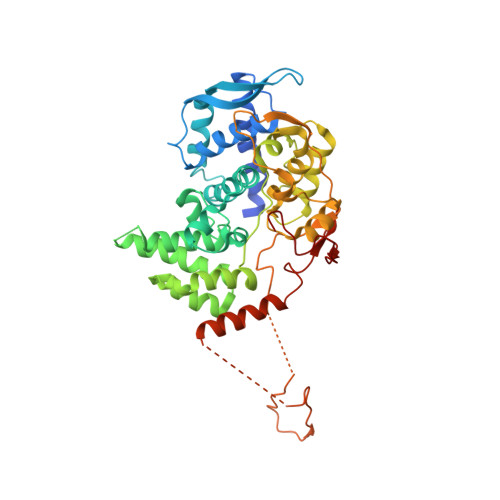
Structural characterization of Thogoto Virus nucleoprotein provides insights into viral RNA encapsidation and RNP assembly.
Dick, A., Mikirtumov, V., Fuchs, J., Krupp, F., Olal, D., Bendl, E., Sprink, T., Diebolder, C., Kudryashev, M., Kochs, G., Roske, Y., Daumke, O.(2024) Structure
- PubMed: 38749445
- DOI: https://doi.org/10.1016/j.str.2024.04.016
- Primary Citation of Related Structures:
8CJW, 8RYT - PubMed Abstract:
Orthomyxoviruses, such as influenza and thogotoviruses, are important human and animal pathogens. Their segmented viral RNA genomes are wrapped by viral nucleoproteins (NPs) into helical ribonucleoprotein complexes (RNPs). NP structures of several influenza viruses have been reported. However, there are still contradictory models of how orthomyxovirus RNPs are assembled. Here, we characterize the crystal structure of Thogoto virus (THOV) NP and found striking similarities to structures of influenza viral NPs, including a two-lobed domain architecture, a positively charged RNA-binding cleft, and a tail loop important for trimerization and viral transcription. A low-resolution cryo-electron tomography reconstruction of THOV RNPs elucidates a left-handed double helical assembly. By providing a model for RNP assembly of THOV, our study suggests conserved NP assembly and RNA encapsidation modes for thogoto- and influenza viruses.
Organizational Affiliation:
From Structural Biology, Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Robert-Rössle-Straße 10, 13125 Berlin, Germany; Institute of Chemistry and Biochemistry, Freie Universität Berlin, Takustraße 6, 14195 Berlin, Germany.