Rapid small-scale nanobody-assisted purification of ryanodine receptors for cryo-EM.
Li, C., Willegems, K., Uchanski, T., Pardon, E., Steyaert, J., Efremov, R.G.(2024) J Biol Chem 300: 107734-107734
- PubMed: 39233227
- DOI: https://doi.org/10.1016/j.jbc.2024.107734
- Primary Citation of Related Structures:
8RRS, 8RRT, 8RRU, 8RRV, 8RRW, 8RRX, 8RS0 - PubMed Abstract:
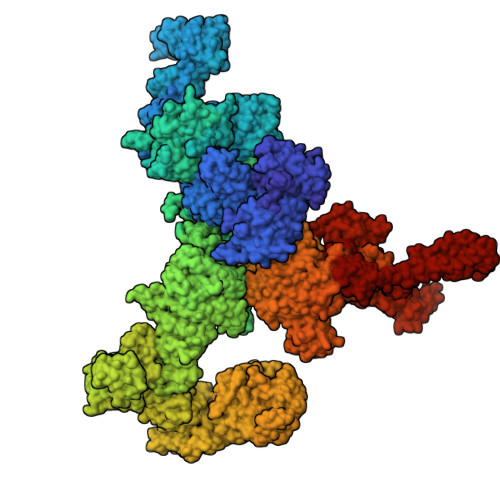
Ryanodine receptors (RyRs) are large Ca 2+ release channels residing in the endoplasmic or sarcoplasmic reticulum membrane. Three isoforms of RyRs have been identified in mammals, the disfunction of which has been associated with a series of life-threatening diseases. The need for large amounts of native tissue or eukaryotic cell cultures limits advances in structural studies of RyRs. Here, we report a method that utilizes nanobodies to purify RyRs from only 5 mg of total protein. The purification process, from isolated membranes to cryo-EM grade protein, is achieved within 4 h on the bench, yielding protein usable for cryo-EM analysis. This is demonstrated by solving the structures of rabbit RyR1, solubilized in detergent, reconstituted into lipid nanodiscs or liposomes, and bovine RyR2 reconstituted in nanodisc, and mouse RyR2 in detergent. The reported method facilitates structural studies of RyRs directed toward drug development and is useful in cases where the amount of starting material is limited.
Organizational Affiliation:
Center for Structural Biology, Vlaams Instituut voor Biotechnologie, Brussels, Belgium; Structural Biology Brussels, Department of Bioengineering Sciences, VUB, Brussels, Belgium.