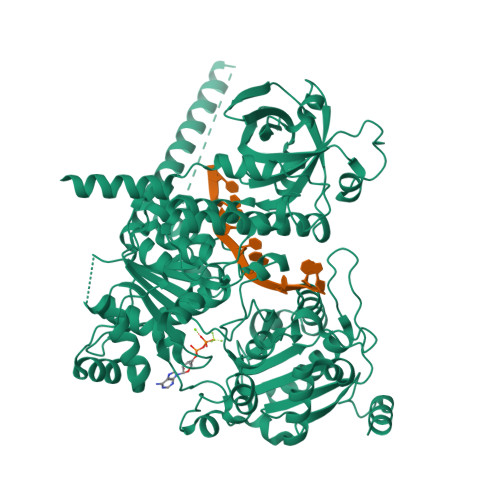
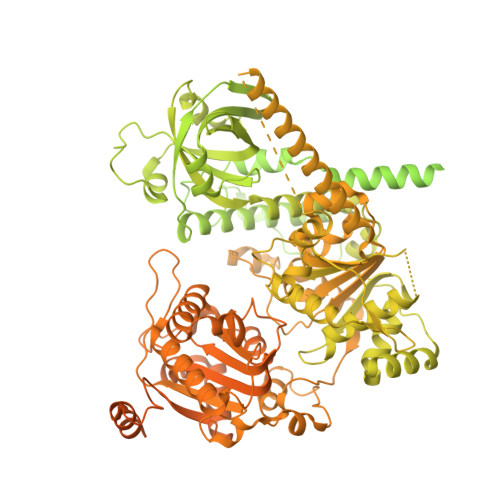
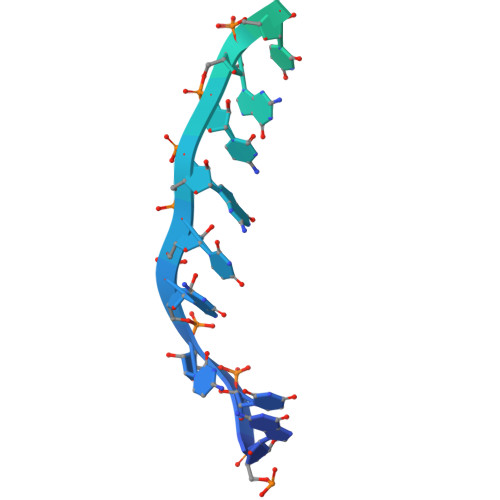
Mechanism of polyadenylation-independent RNA polymerase II termination.
Rengachari, S., Hainthaler, T., Oberthuer, C., Lidschreiber, M., Cramer, P.(2025) Nat Struct Mol Biol 32: 339-345
- PubMed: 39424994
- DOI: https://doi.org/10.1038/s41594-024-01409-0
- Primary Citation of Related Structures:
8RAM, 8RAN, 8RAO, 8RAP - PubMed Abstract:
The mechanisms underlying the initiation and elongation of RNA polymerase II (Pol II) transcription are well-studied, whereas termination remains poorly understood. Here we analyze the mechanism of polyadenylation-independent Pol II termination mediated by the yeast Sen1 helicase. Cryo-electron microscopy structures of two pretermination intermediates show that Sen1 binds to Pol II and uses its adenosine triphosphatase activity to pull on exiting RNA in the 5' direction. This is predicted to push Pol II forward, induce an unstable hypertranslocated state and destabilize the transcription bubble, thereby facilitating termination. This mechanism of transcription termination may be widely used because it is conceptually conserved in the bacterial transcription system.
Organizational Affiliation:
Department of Molecular Biology, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany. srinivasan.rengachari@mpinat.mpg.de.