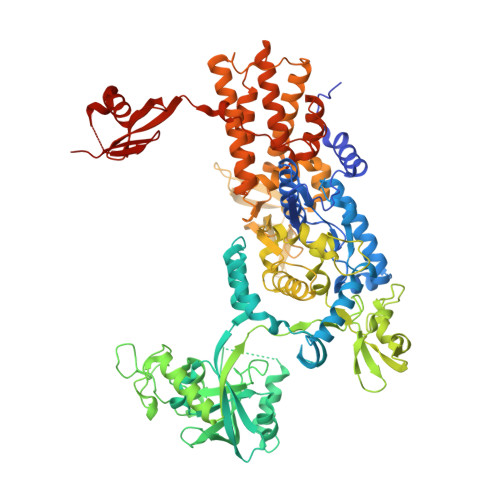
Targeting a microbiota Wolbachian aminoacyl-tRNA synthetase to block its pathogenic host.
Hoffmann, G., Lukarska, M., Clare, R.H., Masters, E.K.G., Johnston, K.L., Ford, L., Turner, J.D., Ward, S.A., Taylor, M.J., Jensen, M.R., Palencia, A.(2024) Sci Adv 10: eado1453-eado1453
- PubMed: 38985862
- DOI: https://doi.org/10.1126/sciadv.ado1453
- Primary Citation of Related Structures:
8POQ, 8POR, 8POS, 8POT - PubMed Abstract:
The interplay between humans and their microbiome is crucial for various physiological processes, including nutrient absorption, immune defense, and maintaining homeostasis. Microbiome alterations can directly contribute to diseases or heighten their likelihood. This relationship extends beyond humans; microbiota play vital roles in other organisms, including eukaryotic pathogens causing severe diseases. Notably, Wolbachia , a bacterial microbiota, is essential for parasitic worms responsible for lymphatic filariasis and onchocerciasis, devastating human illnesses. Given the lack of rapid cures for these infections and the limitations of current treatments, new drugs are imperative. Here, we disrupt Wolbachia 's symbiosis with pathogens using boron-based compounds targeting an unprecedented Wolbachia enzyme, leucyl-tRNA synthetase (LeuRS), effectively inhibiting its growth. Through a compound demonstrating anti- Wolbachia efficacy in infected cells, we use biophysical experiments and x-ray crystallography to elucidate the mechanism behind Wolbachia LeuRS inhibition. We reveal that these compounds form adenosine-based adducts inhibiting protein synthesis. Overall, our study underscores the potential of disrupting key microbiota to control infections.
Organizational Affiliation:
Institute for Advanced Biosciences (IAB), Structural Biology of Novel Drug Targets in Human Diseases, INSERM U1209, CNRS UMR 5309, Université Grenoble-Alpes, Grenoble 38000, France.