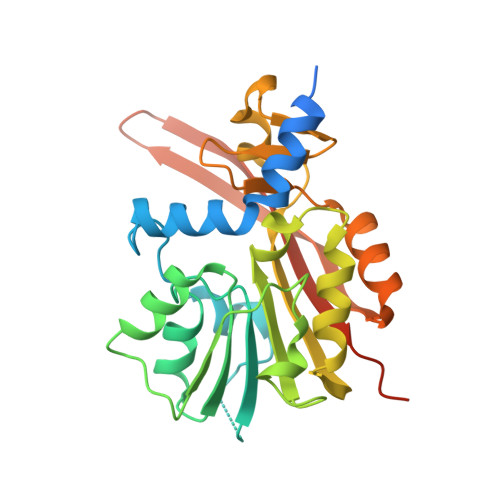
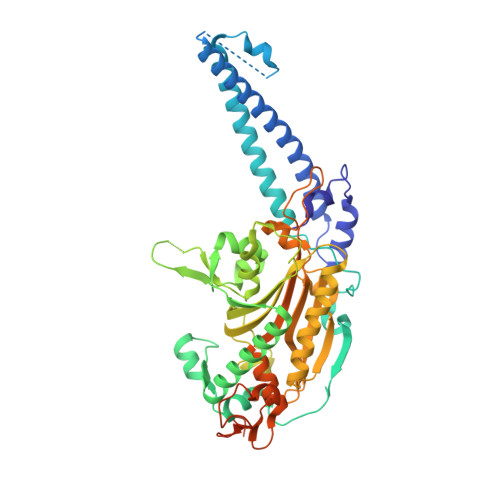
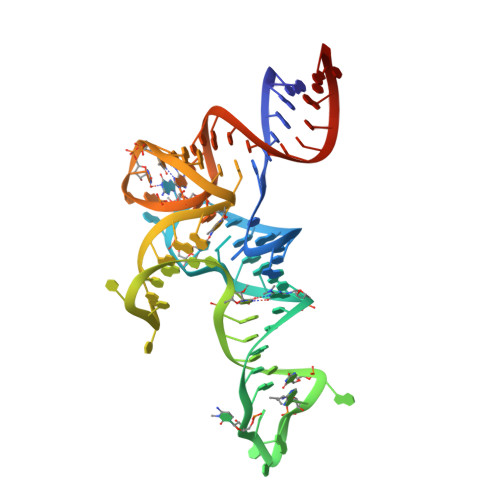
Structural basis of tRNA recognition by the m 3 C RNA methyltransferase METTL6 in complex with SerRS seryl-tRNA synthetase.
Throll, P., G Dolce, L., Rico-Lastres, P., Arnold, K., Tengo, L., Basu, S., Kaiser, S., Schneider, R., Kowalinski, E.(2024) Nat Struct Mol Biol 31: 1614-1624
- PubMed: 38918637
- DOI: https://doi.org/10.1038/s41594-024-01341-3
- Primary Citation of Related Structures:
8OWX, 8OWY, 8P7B, 8P7C, 8P7D - PubMed Abstract:
Methylation of cytosine 32 in the anticodon loop of tRNAs to 3-methylcytosine (m 3 C) is crucial for cellular translation fidelity. Misregulation of the RNA methyltransferases setting this modification can cause aggressive cancers and metabolic disturbances. Here, we report the cryo-electron microscopy structure of the human m 3 C tRNA methyltransferase METTL6 in complex with seryl-tRNA synthetase (SerRS) and their common substrate tRNA Ser . Through the complex structure, we identify the tRNA-binding domain of METTL6. We show that SerRS acts as the tRNA Ser substrate selection factor for METTL6. We demonstrate that SerRS augments the methylation activity of METTL6 and that direct contacts between METTL6 and SerRS are necessary for efficient tRNA Ser methylation. Finally, on the basis of the structure of METTL6 in complex with SerRS and tRNA Ser , we postulate a universal tRNA-binding mode for m 3 C RNA methyltransferases, including METTL2 and METTL8, suggesting that these mammalian paralogs use similar ways to engage their respective tRNA substrates and cofactors.
Organizational Affiliation:
European Molecular Biology Laboratory, Grenoble, France.