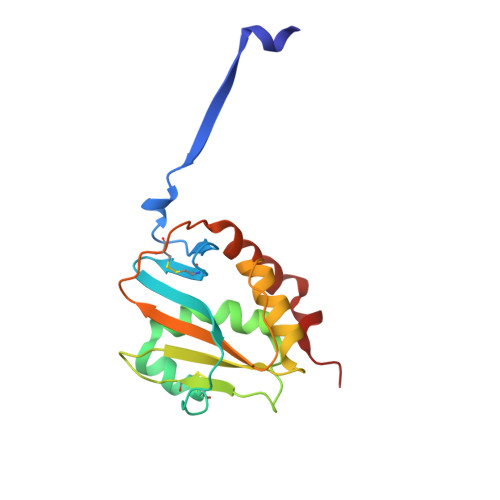
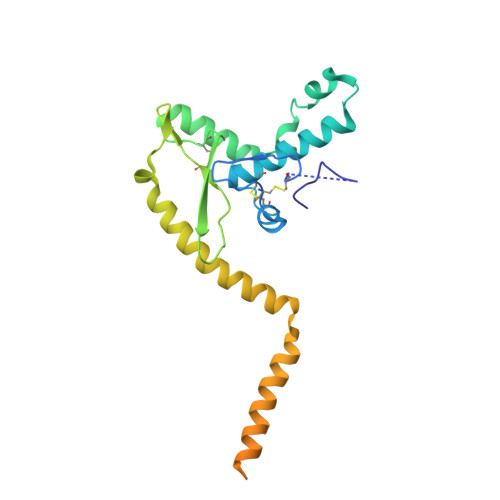
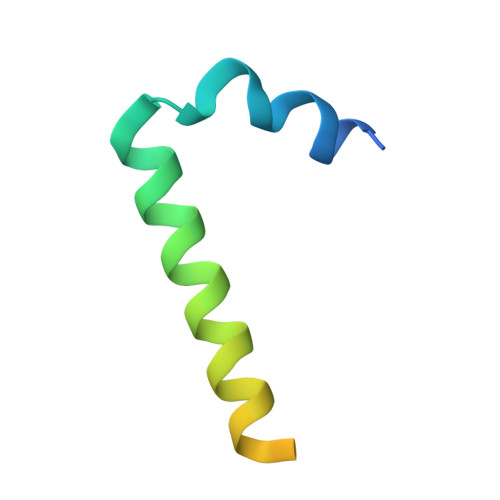
The structure of the Lujo virus spike complex.
Eilon-Ashkenazy, M., Cohen-Dvashi, H., Borni, S., Shaked, R., Calinsky, R., Levy, Y., Diskin, R.(2024) Nat Commun 15: 7175-7175
- PubMed: 39169025
- DOI: https://doi.org/10.1038/s41467-024-51606-0
- Primary Citation of Related Structures:
8P4T - PubMed Abstract:
Lujo virus (LUJV) is a human pathogen that was the cause of a deadly hemorrhagic fever outbreak in Africa. LUJV is a divergent member of the Arenaviridae with some similarities to both the "Old World" and "New World" serogroups, but it uses a cell-entry receptor, neuropilin-2 (NRP2), that is distinct from the receptors of OW and NW viruses. Though the receptor binding domain of LUJV has been characterized structurally, the overall organization of the trimeric spike complex and how NRP2 is recognized in this context were unknown. Here, we present the structure of the membrane-embedded LUJV spike complex determined by cryo-electron microscopy. Analysis of the structure suggested that a single NRP2 molecule is bound at the apex of the trimeric spike and that multiple subunits of the trimer contact the receptor. The binding of NRP2 involves an intriguing arginine-methionine interaction, which we analyzed using quantum mechanical modeling methods. We compare the LUJV spike structure with the only other available structure of a complete arenaviral spike, which is the Lassa virus. The similarities and differences between them shed light on Arenavirus evolution, inform vaccine design, and provide information that will be useful in combating future Arenavirus outbreaks.
Organizational Affiliation:
Department of Chemical and Structural Biology, Weizmann Institute of Science, Rehovot, Israel.