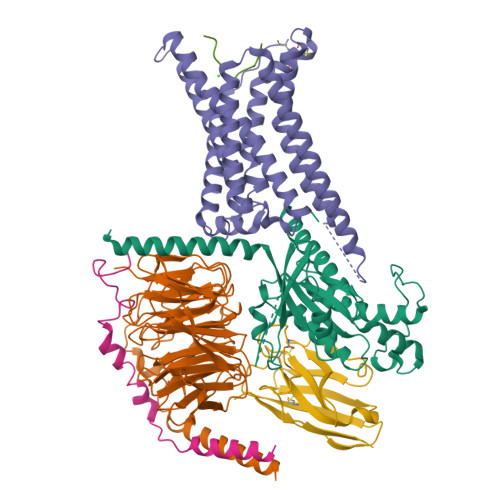
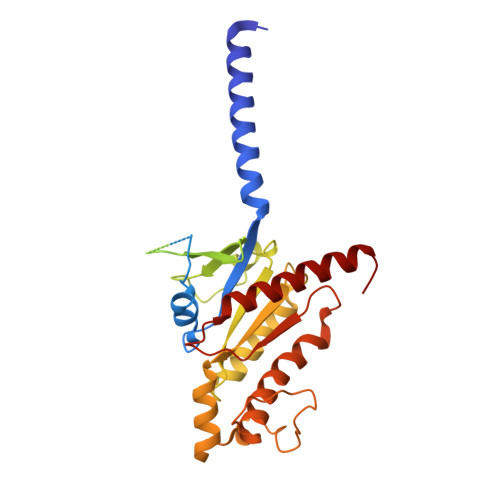
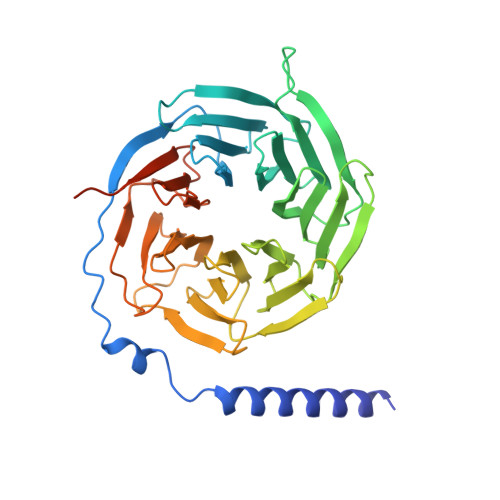
Structural insights into ligand recognition and subtype selectivity of the human melanocortin-3 and melanocortin-5 receptors.
Feng, W., Zhou, Q., Chen, X., Dai, A., Cai, X., Liu, X., Zhao, F., Chen, Y., Ye, C., Xu, Y., Cong, Z., Li, H., Lin, S., Yang, D., Wang, M.W.(2023) Cell Discov 9: 81
- PubMed: 37524700
- DOI: https://doi.org/10.1038/s41421-023-00586-4
- Primary Citation of Related Structures:
8INR, 8IOC, 8IOD - PubMed Abstract:
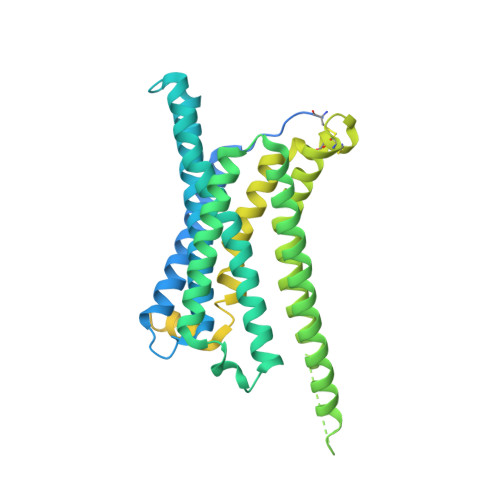
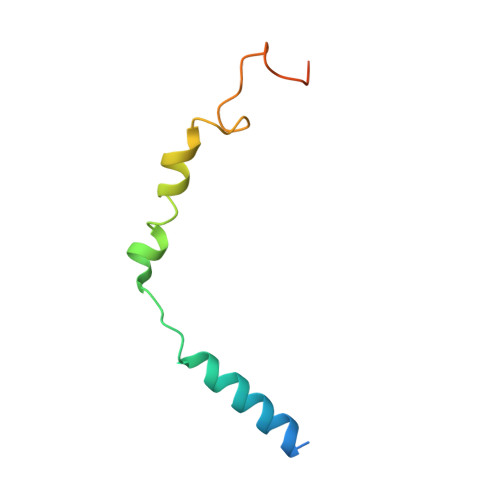
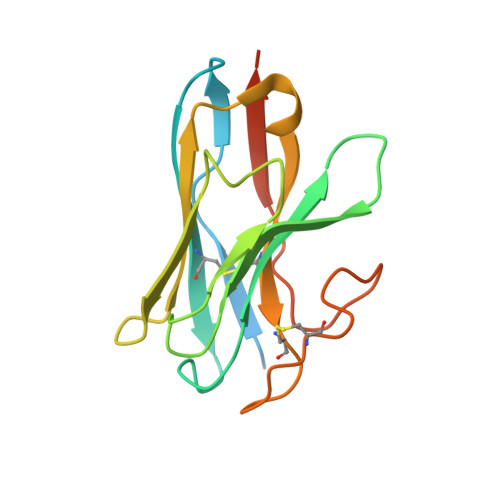
Members of the melanocortin receptor (MCR) family that recognize different melanocortin peptides mediate a broad spectrum of cellular processes including energy homeostasis, inflammation and skin pigmentation through five MCR subtypes (MC1R-MC5R). The structural basis of subtype selectivity of the endogenous agonist γ-MSH and non-selectivity of agonist α-MSH remains elusive, as the two agonists are highly similar with a conserved HFRW motif. Here, we report three cryo-electron microscopy structures of MC3R-G s in complex with γ-MSH and MC5R-G s in the presence of α-MSH or a potent synthetic agonist PG-901. The structures reveal that α-MSH and γ-MSH adopt a "U-shape" conformation, penetrate into the wide-open orthosteric pocket and form massive common contacts with MCRs via the HFRW motif. The C-terminus of γ-MSH occupies an MC3R-specific complementary binding groove likely conferring subtype selectivity, whereas that of α-MSH distances itself from the receptor with neglectable contacts. PG-901 achieves the same potency as α-MSH with a shorter length by rebalancing the recognition site and mimicking the intra-peptide salt bridge in α-MSH by cyclization. Solid density confirmed the calcium ion binding in MC3R and MC5R, and the distinct modulation effects of divalent ions were demonstrated. Our results provide insights into ligand recognition and subtype selectivity among MCRs, and expand the knowledge of signal transduction among MCR family members.
Organizational Affiliation:
Department of Pharmacology, School of Basic Medical Sciences, Fudan University, Shanghai, China.