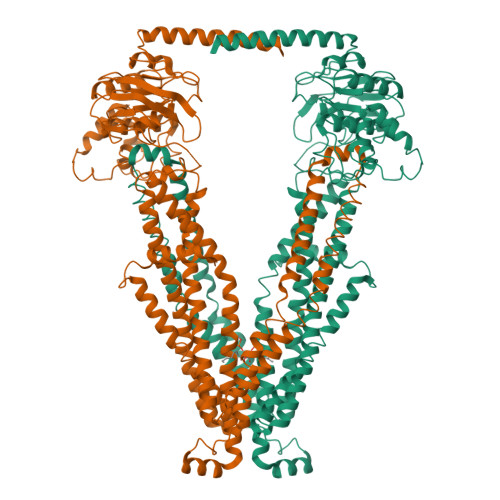
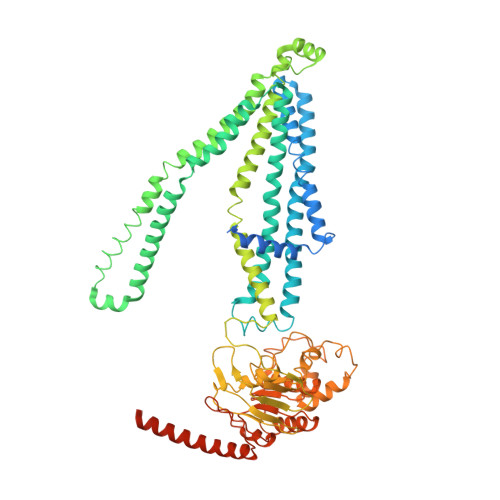
Structural insights into substrate recognition and translocation of human peroxisomal ABC transporter ALDP.
Xiong, C., Jia, L.N., Xiong, W.X., Wu, X.T., Xiong, L.L., Wang, T.H., Zhou, D., Hong, Z., Liu, Z., Tang, L.(2023) Signal Transduct Target Ther 8: 74-74
- PubMed: 36810450
- DOI: https://doi.org/10.1038/s41392-022-01280-9
- Primary Citation of Related Structures:
7X07, 7X0T, 7X0Z, 7X1W, 7XEC, 7YRQ - PubMed Abstract:
Dysfunctions of ATP-binding cassette, subfamily D, member 1 (ABCD1) cause X-linked adrenoleukodystrophy, a rare neurodegenerative disease that affects all human tissues. Residing in the peroxisome membrane, ABCD1 plays a role in the translocation of very long-chain fatty acids for their β-oxidation. Here, the six cryo-electron microscopy structures of ABCD1 in four distinct conformational states were presented. In the transporter dimer, two transmembrane domains form the substrate translocation pathway, and two nucleotide-binding domains form the ATP-binding site that binds and hydrolyzes ATP. The ABCD1 structures provide a starting point for elucidating the substrate recognition and translocation mechanism of ABCD1. Each of the four inward-facing structures of ABCD1 has a vestibule that opens to the cytosol with variable sizes. Hexacosanoic acid (C26:0)-CoA substrate binds to the transmembrane domains (TMDs) and stimulates the ATPase activity of the nucleotide-binding domains (NBDs). W339 from the transmembrane helix 5 (TM5) is essential for binding substrate and stimulating ATP hydrolysis by substrate. ABCD1 has a unique C-terminal coiled-coil domain that negatively modulates the ATPase activity of the NBDs. Furthermore, the structure of ABCD1 in the outward-facing state indicates that ATP molecules pull the two NBDs together and open the TMDs to the peroxisomal lumen for substrate release. The five structures provide a view of the substrate transport cycle and mechanistic implication for disease-causing mutations.
Organizational Affiliation:
Department of Neurology, State Key Lab of Biotherapy and Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center for Biotherapy, 610041, Chengdu, Sichuan, China.