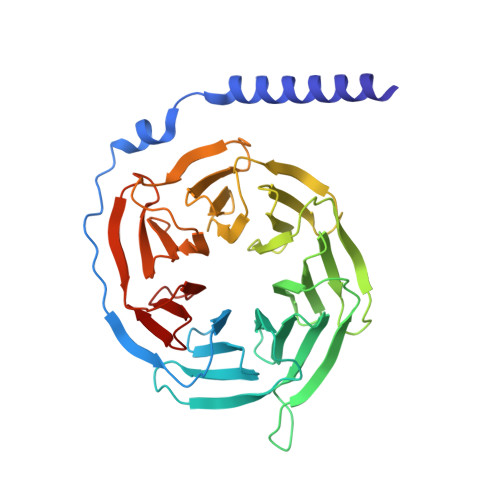
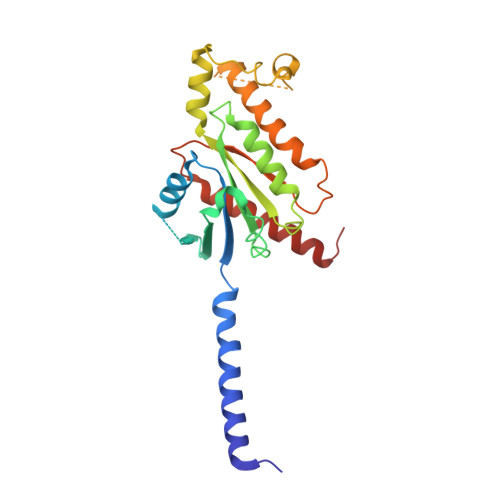
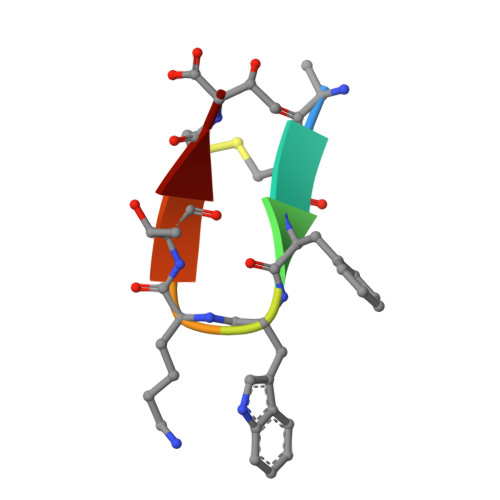
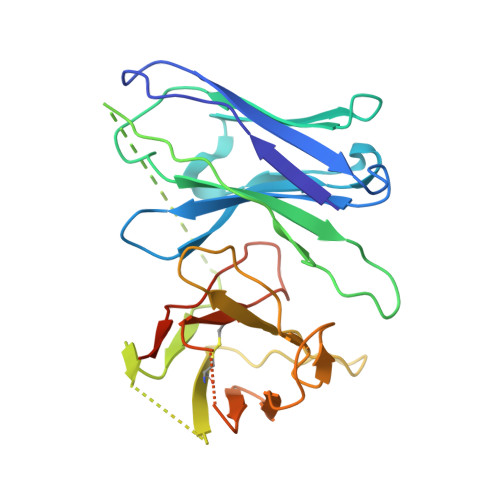
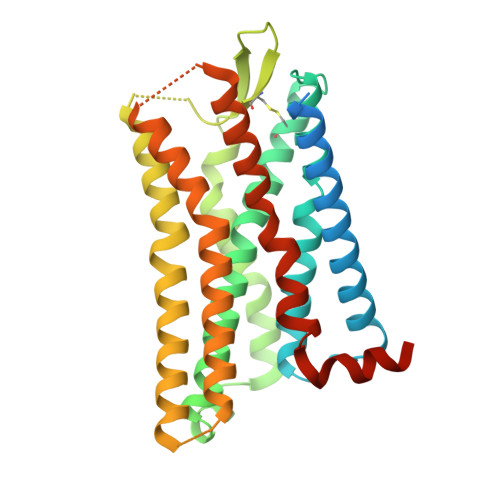
Molecular basis for the selective G protein signaling of somatostatin receptors.
Chen, S., Teng, X., Zheng, S.(2023) Nat Chem Biol 19: 133-140
- PubMed: 36138141
- DOI: https://doi.org/10.1038/s41589-022-01130-3
- Primary Citation of Related Structures:
7Y24, 7Y26, 7Y27 - PubMed Abstract:
G protein-coupled receptors (GPCRs) modulate every aspect of physiological functions mainly through activating heterotrimeric G proteins. A majority of GPCRs promiscuously couple to multiple G protein subtypes. Here we validate that in addition to the well-known G i/o pathway, somatostatin receptor 2 and 5 (SSTR2 and SSTR5) couple to the G q/11 pathway and show that smaller ligands preferentially activate the G i/o pathway. We further determined cryo-electron microscopy structures of the SSTR2‒G o and SSTR2‒G q complexes bound to octreotide and SST-14. Structural and functional analysis revealed that G protein selectivity of SSTRs is not only determined by structural elements in the receptor-G protein interface, but also by the conformation of the agonist-binding pocket. Accordingly, smaller ligands fail to stabilize a broader agonist-binding pocket of SSTRs that is required for efficient G q/11 coupling but not G i/o coupling. Our studies facilitate the design of drugs with selective G protein signaling to improve therapeutic efficacy.
Organizational Affiliation:
National Institute of Biological Sciences, Beijing, China.