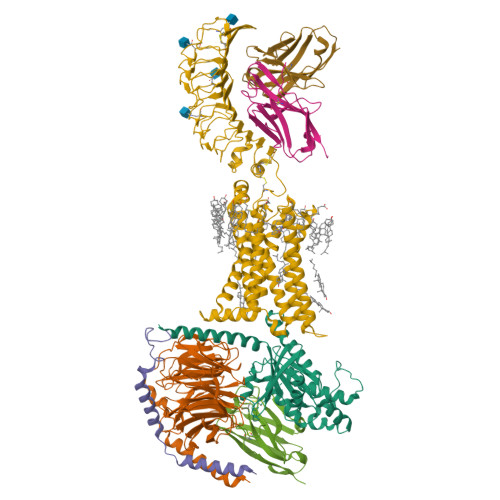
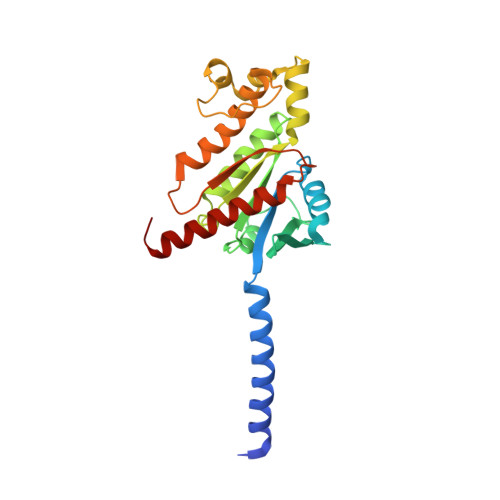
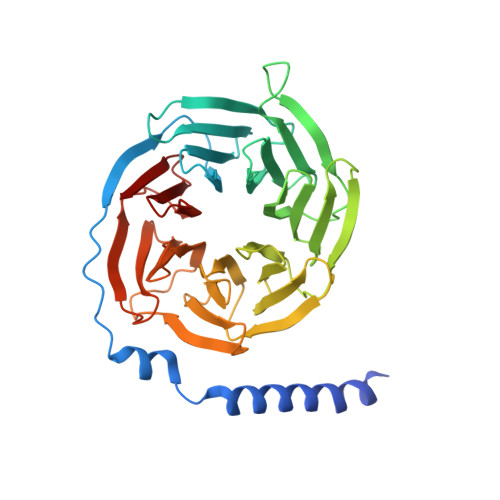
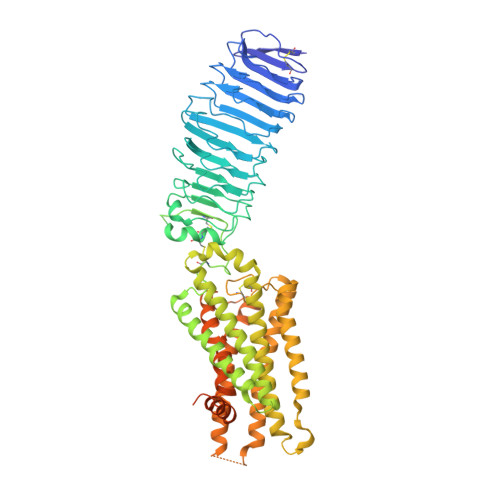
Hormone- and antibody-mediated activation of the thyrotropin receptor.
Duan, J., Xu, P., Luan, X., Ji, Y., He, X., Song, N., Yuan, Q., Jin, Y., Cheng, X., Jiang, H., Zheng, J., Zhang, S., Jiang, Y., Xu, H.E.(2022) Nature 609: 854-859
- PubMed: 35940204
- DOI: https://doi.org/10.1038/s41586-022-05173-3
- Primary Citation of Related Structures:
7XW5, 7XW6, 7XW7 - PubMed Abstract:
Thyroid-stimulating hormone (TSH), through activation of its G-protein-coupled thyrotropin receptor (TSHR), controls the synthesis of thyroid hormone-an essential metabolic hormone 1-3 . Aberrant signalling of TSHR by autoantibodies causes Graves' disease (hyperthyroidism) and hypothyroidism, both of which affect millions of patients worldwide 4 . Here we report the active structures of TSHR with TSH and the activating autoantibody M22 5 , both bound to the allosteric agonist ML-109 6 , as well as an inactivated TSHR structure with the inhibitory antibody K1-70 7 . Both TSH and M22 push the extracellular domain (ECD) of TSHR into an upright active conformation. By contrast, K1-70 blocks TSH binding and cannot push the ECD into the upright conformation. Comparisons of the active and inactivated structures of TSHR with those of the luteinizing hormone/choriogonadotropin receptor (LHCGR) reveal a universal activation mechanism of glycoprotein hormone receptors, in which a conserved ten-residue fragment (P10) from the hinge C-terminal loop mediates ECD interactions with the TSHR transmembrane domain 8 . One notable feature is that there are more than 15 cholesterols surrounding TSHR, supporting its preferential location in lipid rafts 9 . These structures also highlight a similar ECD-push mechanism for TSH and autoantibody M22 to activate TSHR, therefore providing the molecular basis for Graves' disease.
Organizational Affiliation:
The CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.