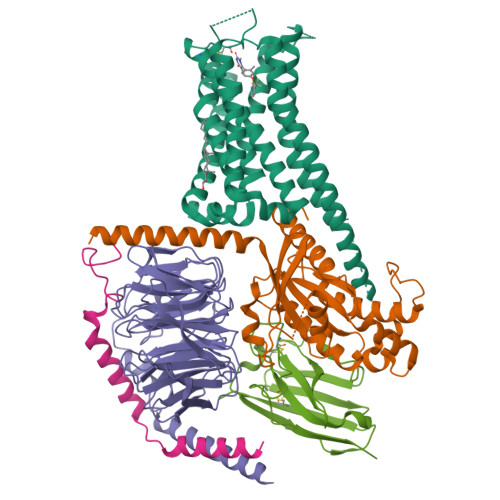
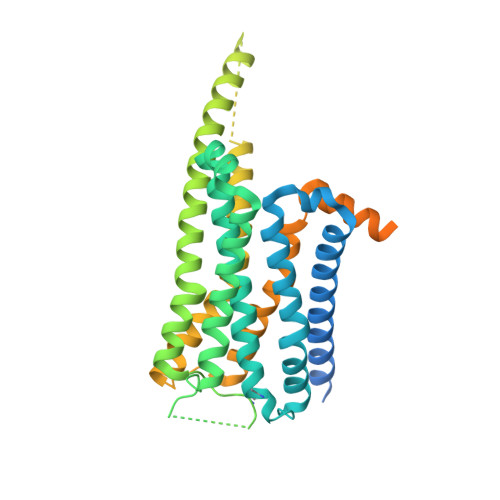
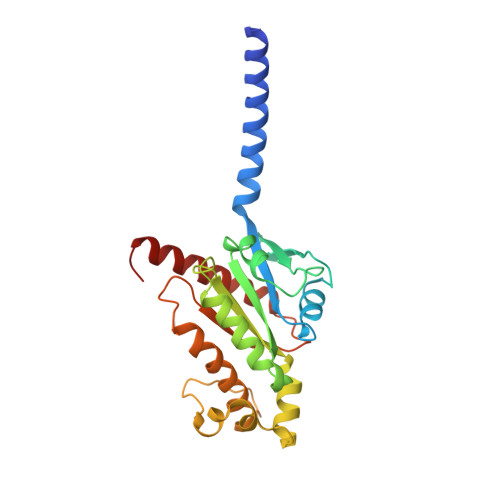
Ligand recognition and biased agonism of the D1 dopamine receptor.
Teng, X., Chen, S., Nie, Y., Xiao, P., Yu, X., Shao, Z., Zheng, S.(2022) Nat Commun 13: 3186-3186
- PubMed: 35676276
- DOI: https://doi.org/10.1038/s41467-022-30929-w
- Primary Citation of Related Structures:
7X2C, 7X2D, 7X2F - PubMed Abstract:
Dopamine receptors are widely distributed in the central nervous system and are important therapeutic targets for treatment of various psychiatric and neurological diseases. Here, we report three cryo-electron microscopy structures of the D1 dopamine receptor (D1R)-Gs complex bound to two agonists, fenoldopam and tavapadon, and a positive allosteric modulator LY3154207. The structure reveals unusual binding of two fenoldopam molecules, one to the orthosteric binding pocket (OBP) and the other to the extended binding pocket (EBP). In contrast, one elongated tavapadon molecule binds to D1R, extending from OBP to EBP. Moreover, LY3154207 stabilizes the second intracellular loop of D1R in an alpha helical conformation to efficiently engage the G protein. Through a combination of biochemical, biophysical and cellular assays, we further show that the broad conformation stabilized by two fenoldopam molecules and interaction between TM5 and the agonist are important for biased signaling of D1R.
Organizational Affiliation:
Tsinghua Institute of Multidisciplinary Biomedical Research, Tsinghua University, Beijing, China.