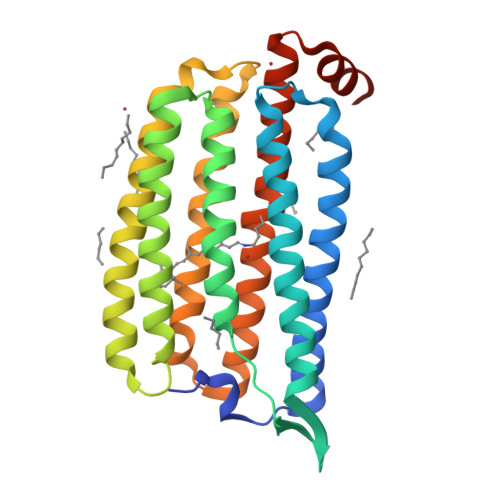
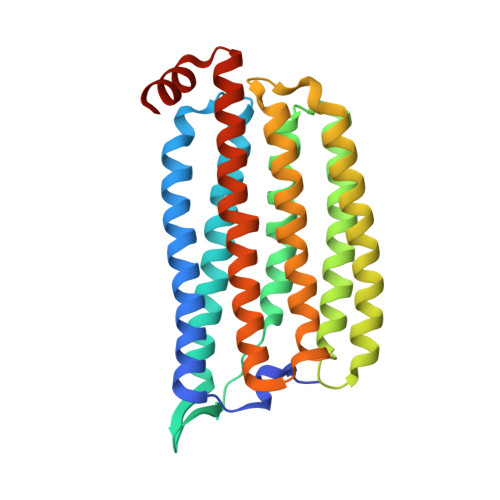
Conformational alterations in unidirectional ion transport of a light-driven chloride pump revealed using X-ray free electron lasers.
Hosaka, T., Nomura, T., Kubo, M., Nakane, T., Fangjia, L., Sekine, S.I., Ito, T., Murayama, K., Ihara, K., Ehara, H., Kashiwagi, K., Katsura, K., Akasaka, R., Hisano, T., Tanaka, T., Tanaka, R., Arima, T., Yamashita, A., Sugahara, M., Naitow, H., Matsuura, Y., Yoshizawa, S., Tono, K., Owada, S., Nureki, O., Kimura-Someya, T., Iwata, S., Nango, E., Shirouzu, M.(2022) Proc Natl Acad Sci U S A 119
- PubMed: 35197289
- DOI: https://doi.org/10.1073/pnas.2117433119
- Primary Citation of Related Structures:
7VGT, 7VGU, 7VGV - PubMed Abstract:
Light-driven chloride-pumping rhodopsins actively transport anions, including various halide ions, across cell membranes. Recent studies using time-resolved serial femtosecond crystallography (TR-SFX) have uncovered the structural changes and ion transfer mechanisms in light-driven cation-pumping rhodopsins. However, the mechanism by which the conformational changes pump an anion to achieve unidirectional ion transport, from the extracellular side to the cytoplasmic side, in anion-pumping rhodopsins remains enigmatic. We have collected TR-SFX data of Nonlabens marinus rhodopsin-3 (NM-R3), derived from a marine flavobacterium, at 10-µs and 1-ms time points after photoexcitation. Our structural analysis reveals the conformational alterations during ion transfer and after ion release. Movements of the retinal chromophore initially displace a conserved tryptophan to the cytoplasmic side of NM-R3, accompanied by a slight shift of the halide ion bound to the retinal. After ion release, the inward movements of helix C and helix G and the lateral displacements of the retinal block access to the extracellular side of NM-R3. Anomalous signal data have also been obtained from NM-R3 crystals containing iodide ions. The anomalous density maps provide insight into the halide binding site for ion transfer in NM-R3.
Organizational Affiliation:
RIKEN Center for Biosystems Dynamics Research, Yokohama, Kanagawa 230-0045, Japan.