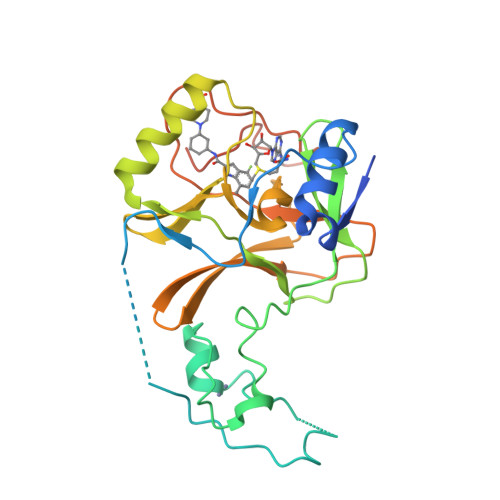
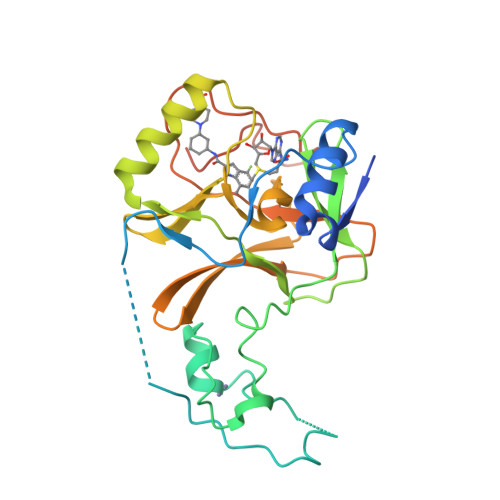
Conformational-Design-Driven Discovery of EZM0414: A Selective, Potent SETD2 Inhibitor for Clinical Studies.
Alford, J.S., Lampe, J.W., Brach, D., Chesworth, R., Cosmopoulos, K., Duncan, K.W., Eckley, S.T., Kutok, J.L., Raimondi, A., Riera, T.V., Shook, B., Tang, C., Totman, J., Farrow, N.A.(2022) ACS Med Chem Lett 13: 1137-1143
- PubMed: 35859865
- DOI: https://doi.org/10.1021/acsmedchemlett.2c00167
- Primary Citation of Related Structures:
7TY2, 7TY3 - PubMed Abstract:
SETD2, a lysine N -methyltransferase, is a histone methyltransferase that plays an important role in various cellular processes and was identified as a target of interest in multiple myeloma that features a t(4,14) translocation. We recently reported the discovery of a novel small-molecule SETD2 inhibitor tool compound that is suitable for preclinical studies. Herein we describe the conformational-design-driven evolution of the advanced chemistry lead, which resulted in compounds appropriate for clinical evaluation. Further optimization of this chemical series led to the discovery of EZM0414, which is a potent, selective, and orally bioavailable inhibitor of SETD2 with good pharmacokinetic properties and robust pharmacodynamic activity in a mouse xenograft model.
Organizational Affiliation:
Epizyme Inc., 50 Hampshire Street, Sixth Floor, Cambridge, Massachusetts 02139, United States.