A lipoprotein allosterically activates the CwlD amidase during Clostridioides difficile spore formation.
Alves Feliciano, C., Eckenroth, B.E., Diaz, O.R., Doublie, S., Shen, A.(2021) PLoS Genet 17: e1009791-e1009791
- PubMed: 34570752
- DOI: https://doi.org/10.1371/journal.pgen.1009791
- Primary Citation of Related Structures:
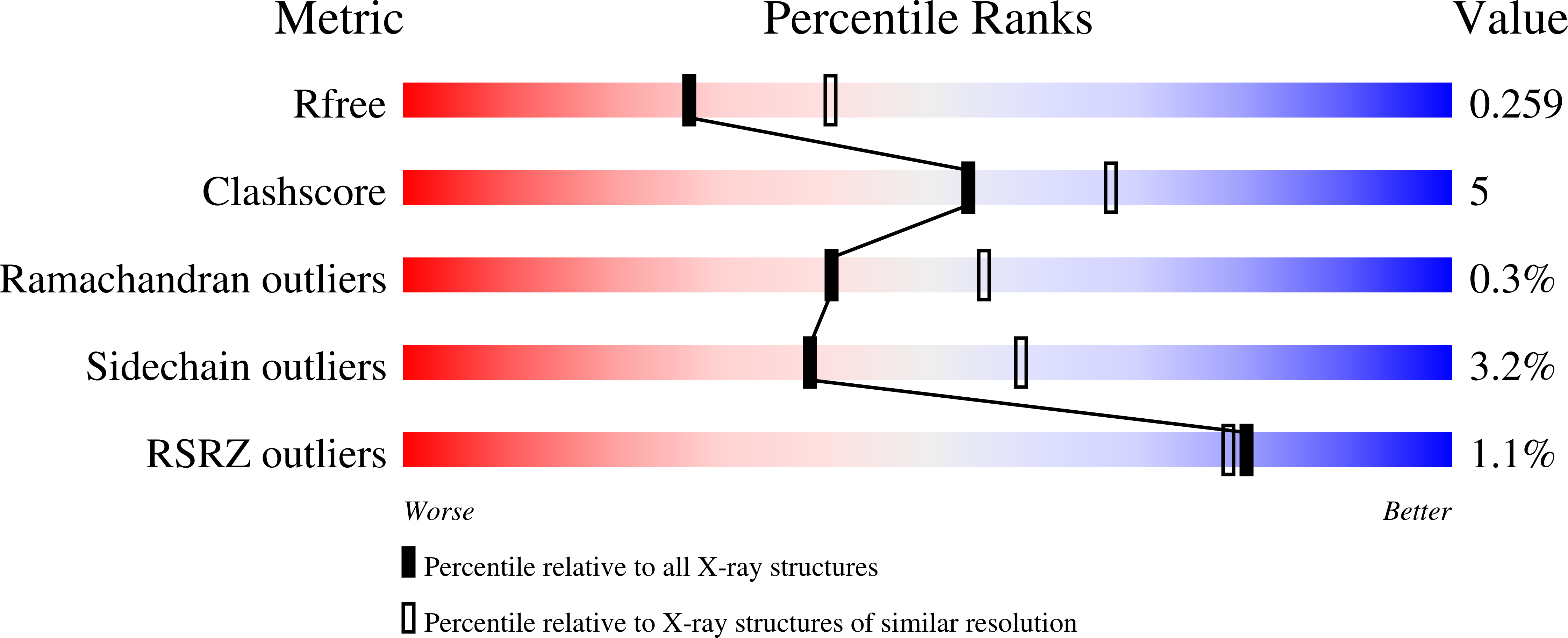
7RAG - PubMed Abstract:
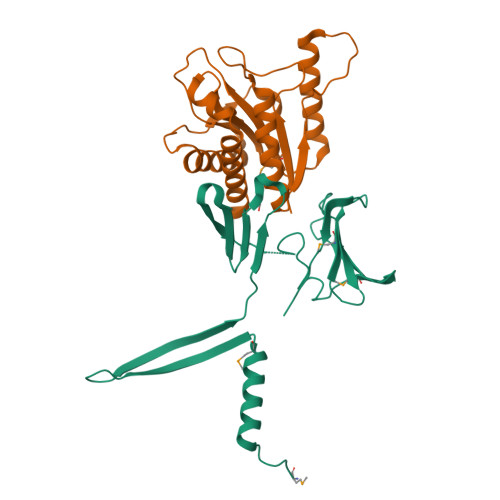
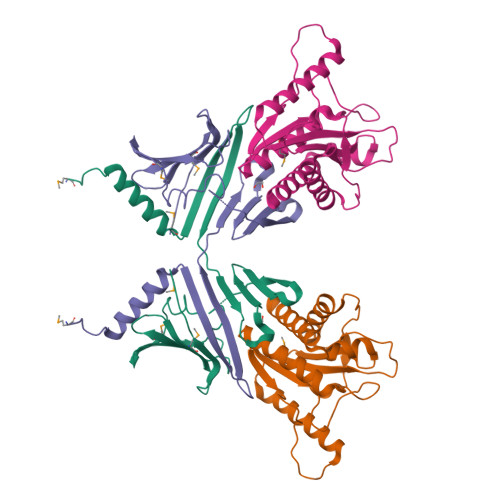
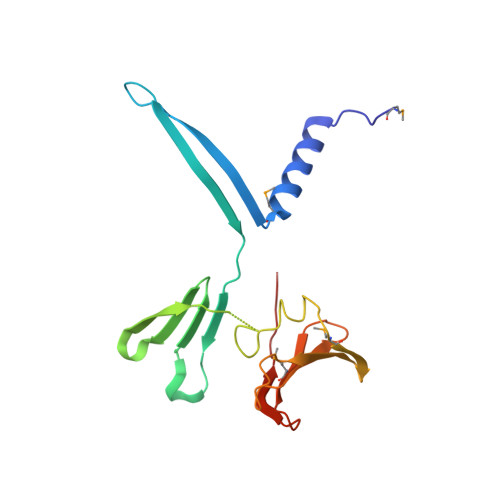
Spore-forming pathogens like Clostridioides difficile depend on germination to initiate infection. During gemination, spores must degrade their cortex layer, which is a thick, protective layer of modified peptidoglycan. Cortex degradation depends on the presence of the spore-specific peptidoglycan modification, muramic-∂-lactam (MAL), which is specifically recognized by cortex lytic enzymes. In C. difficile, MAL production depends on the CwlD amidase and its binding partner, the GerS lipoprotein. To gain insight into how GerS regulates CwlD activity, we solved the crystal structure of the CwlD:GerS complex. In this structure, a GerS homodimer is bound to two CwlD monomers such that the CwlD active sites are exposed. Although CwlD structurally resembles amidase_3 family members, we found that CwlD does not bind Zn2+ stably on its own, unlike previously characterized amidase_3 enzymes. Instead, GerS binding to CwlD promotes CwlD binding to Zn2+, which is required for its catalytic mechanism. Thus, in determining the first structure of an amidase bound to its regulator, we reveal stabilization of Zn2+ co-factor binding as a novel mechanism for regulating bacterial amidase activity. Our results further suggest that allosteric regulation by binding partners may be a more widespread mode for regulating bacterial amidase activity than previously thought.
Organizational Affiliation:
Department of Molecular Biology and Microbiology, Tufts University School of Medicine, Boston, Massachusetts, United States of America.