Discovery and Characterisation of Highly Cooperative FAK-Degrading PROTACs.
Law, R.P., Nunes, J., Chung, C.W., Bantscheff, M., Buda, K., Dai, H., Evans, J.P., Flinders, A., Klimaszewska, D., Lewis, A.J., Muelbaier, M., Scott-Stevens, P., Stacey, P., Tame, C.J., Watt, G.F., Zinn, N., Queisser, M.A., Harling, J.D., Benowitz, A.B.(2021) Angew Chem Int Ed Engl 60: 23327-23334
- PubMed: 34416073
- DOI: https://doi.org/10.1002/anie.202109237
- Primary Citation of Related Structures:
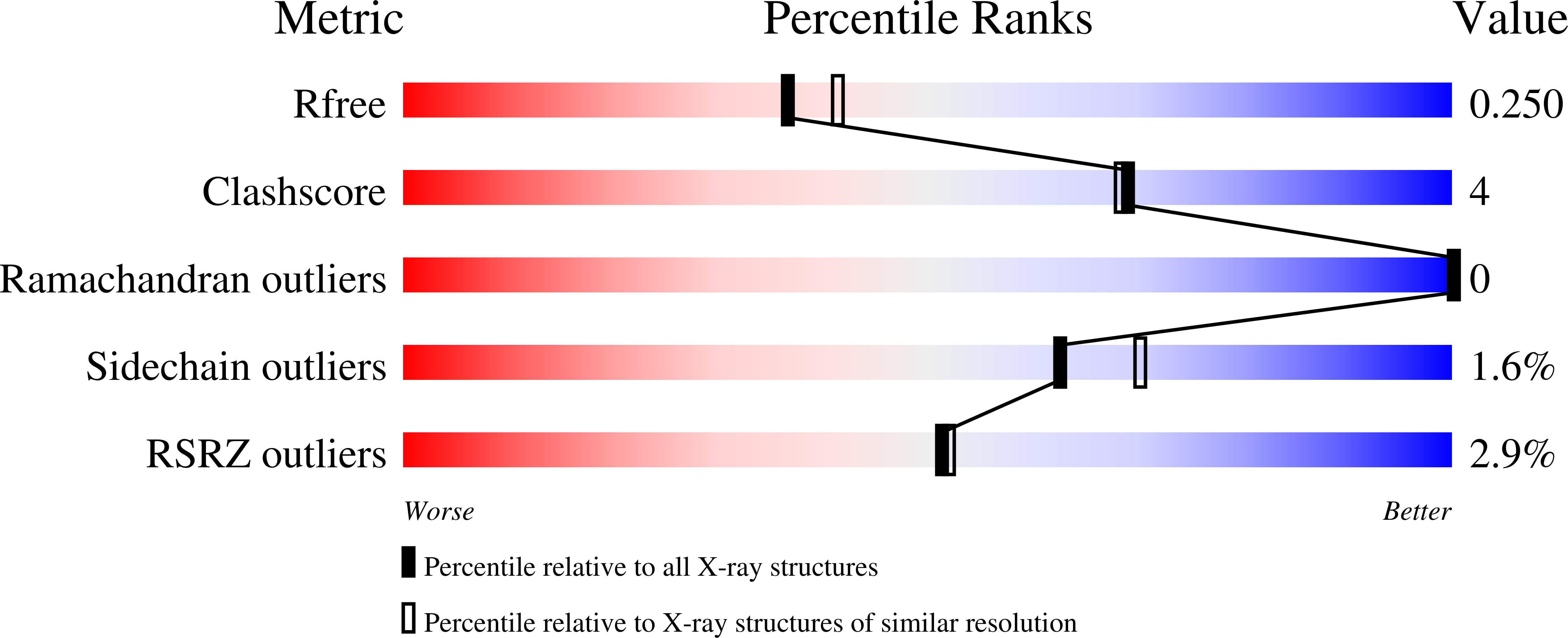
7PI4 - PubMed Abstract:
Focal adhesion kinase (FAK) is a key mediator of tumour progression and metastasis. To date, clinical trials of FAK inhibitors have reported disappointing efficacy for oncology indications. We report the design and characterisation of GSK215, a potent, selective, FAK-degrading Proteolysis Targeting Chimera (PROTAC) based on a binder for the VHL E3 ligase and the known FAK inhibitor VS-4718. X-ray crystallography revealed the molecular basis of the highly cooperative FAK-GSK215-VHL ternary complex, and GSK215 showed differentiated in-vitro pharmacology compared to VS-4718. In mice, a single dose of GSK215 induced rapid and prolonged FAK degradation, giving a long-lasting effect on FAK levels (≈96 h) and a marked PK/PD disconnect. This tool PROTAC molecule is expected to be useful for the study of FAK-degradation biology in vivo, and our results indicate that FAK degradation may be a differentiated clinical strategy versus FAK inhibition for the treatment of cancer.
Organizational Affiliation:
GlaxoSmithKline, Gunnels Wood Road, Stevenage, Hertfordshire, SG1 2NY, UK.