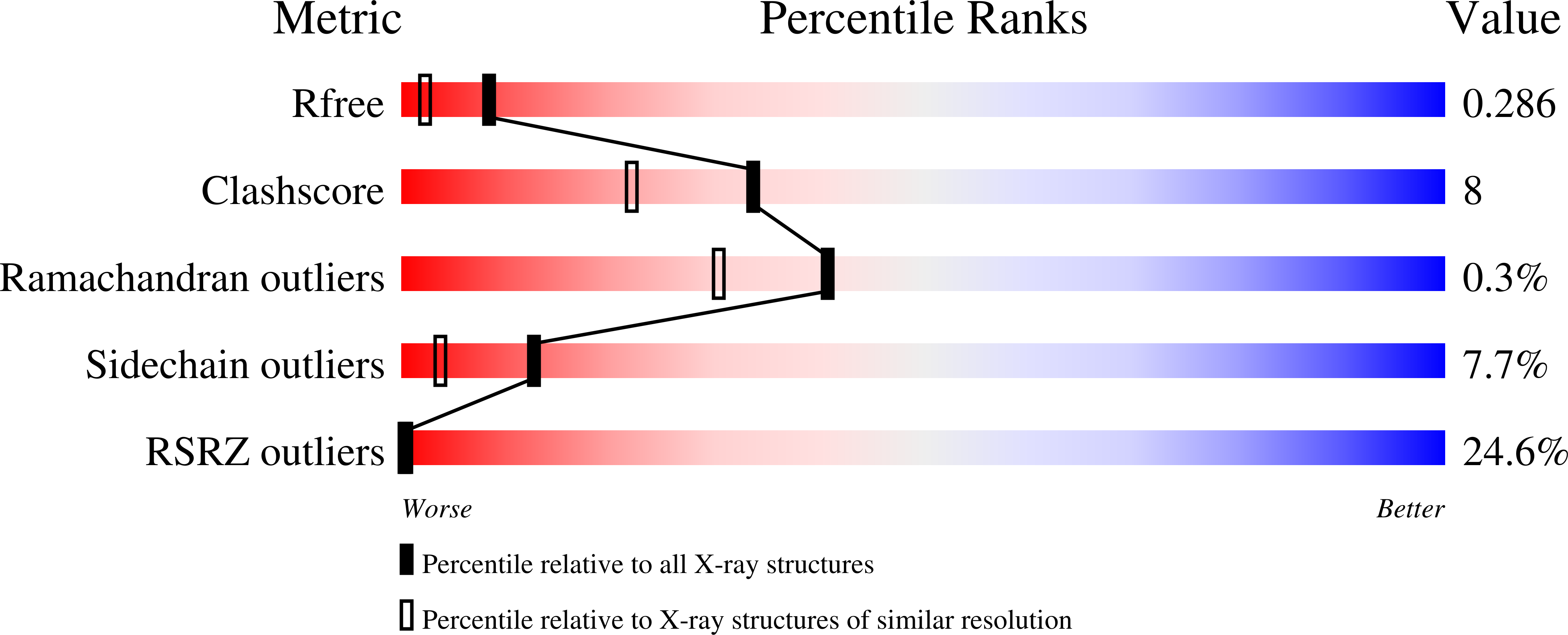
Structural basis for UFM1 transfer from UBA5 to UFC1.
Kumar, M., Padala, P., Fahoum, J., Hassouna, F., Tsaban, T., Zoltsman, G., Banerjee, S., Cohen-Kfir, E., Dessau, M., Rosenzweig, R., Isupov, M.N., Schueler-Furman, O., Wiener, R.(2021) Nat Commun 12: 5708-5708
- PubMed: 34588452
- DOI: https://doi.org/10.1038/s41467-021-25994-6
- Primary Citation of Related Structures:
7NVJ, 7NVK, 7NW1 - PubMed Abstract:
Ufmylation is a post-translational modification essential for regulating key cellular processes. A three-enzyme cascade involving E1, E2 and E3 is required for UFM1 attachment to target proteins. How UBA5 (E1) and UFC1 (E2) cooperatively activate and transfer UFM1 is still unclear. Here, we present the crystal structure of UFC1 bound to the C-terminus of UBA5, revealing how UBA5 interacts with UFC1 via a short linear sequence, not observed in other E1-E2 complexes. We find that UBA5 has a region outside the adenylation domain that is dispensable for UFC1 binding but critical for UFM1 transfer. This region moves next to UFC1's active site Cys and compensates for a missing loop in UFC1, which exists in other E2s and is needed for the transfer. Overall, our findings advance the understanding of UFM1's conjugation machinery and may serve as a basis for the development of ufmylation inhibitors.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, The Institute for Medical Research Israel-Canada, Hebrew University-Hadassah Medical School, Jerusalem, 91120, Israel.