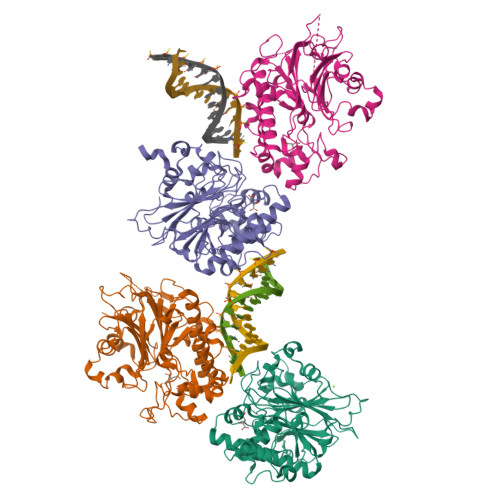
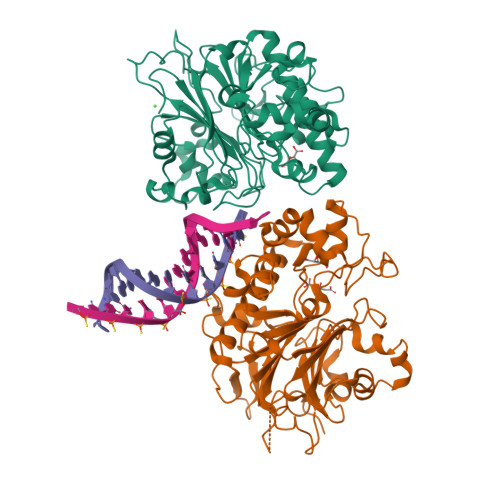
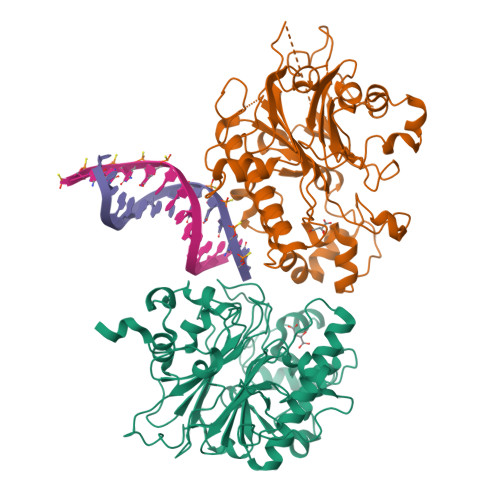
Molecular basis for processing of topoisomerase 1-triggered DNA damage by Apn2/APE2.
Williams, J.S., Wojtaszek, J.L., Appel, D.C., Krahn, J., Wallace, B.D., Walsh, E., Kunkel, T.A., Williams, R.S.(2022) Cell Rep 41: 111448-111448
- PubMed: 36198268
- DOI: https://doi.org/10.1016/j.celrep.2022.111448
- Primary Citation of Related Structures:
7N3Y, 7N3Z - PubMed Abstract:
Topoisomerase 1 (Top1) incises DNA containing ribonucleotides to generate complex DNA lesions that are resolved by APE2 (Apn2 in yeast). How Apn2 engages and processes this DNA damage is unclear. Here, we report X-ray crystal structures and biochemical analysis of Apn2-DNA complexes to demonstrate how Apn2 frays and cleaves 3' DNA termini via a wedging mechanism that facilitates 1-6 nucleotide endonucleolytic cleavages. APN2 deletion and DNA-wedge mutant Saccharomyces cerevisiae strains display mutator phenotypes, cell growth defects, and sensitivity to genotoxic stress in a ribonucleotide excision repair (RER)-defective background harboring a high density of Top1-incised ribonucleotides. Our data implicate a wedge-and-cut mechanism underpinning the broad-specificity Apn2 nuclease activity that mitigates mutagenic and genome instability phenotypes caused by Top1 incision at genomic ribonucleotides incorporated by DNA polymerase epsilon.
Organizational Affiliation:
Genome Integrity and Structural Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, US Department of Health and Human Services, Research Triangle Park, NC 27709, USA.