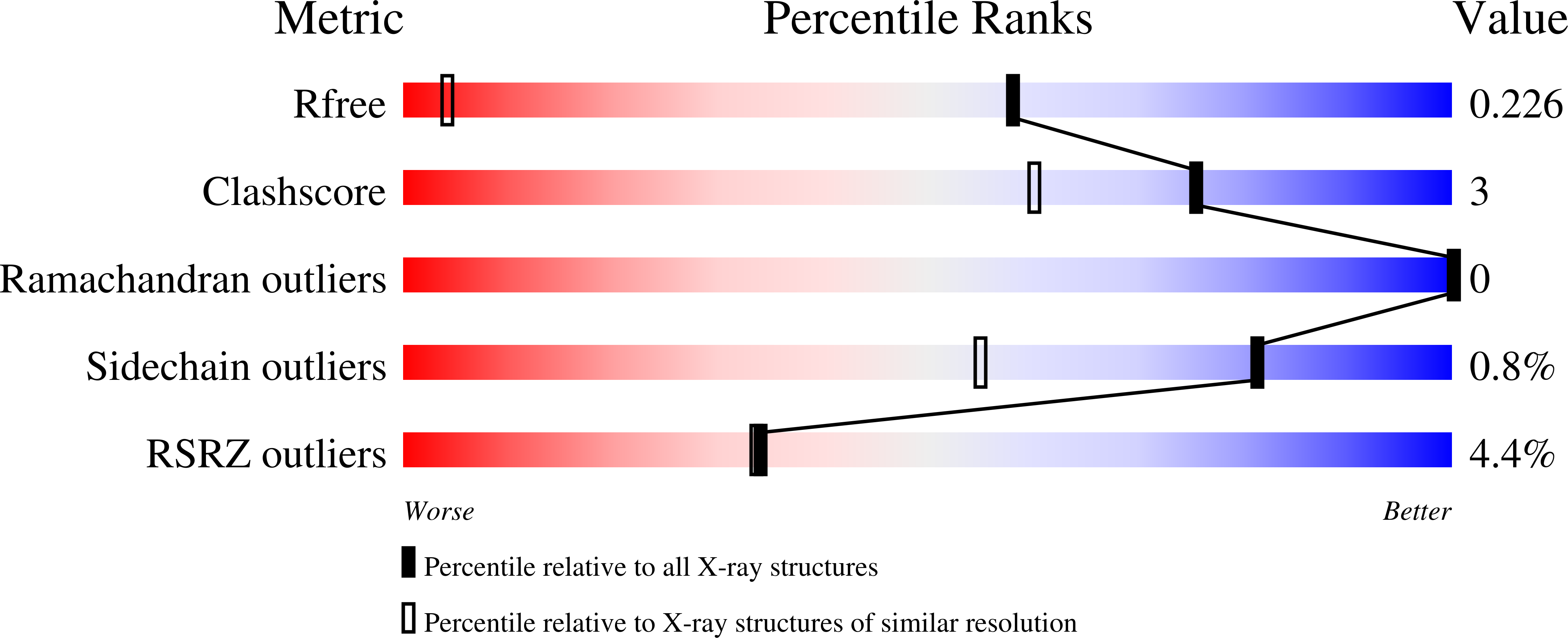
Energy penalties enhance flexible receptor docking in a model cavity.
Kamenik, A.S., Singh, I., Lak, P., Balius, T.E., Liedl, K.R., Shoichet, B.K.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34475217
- DOI: https://doi.org/10.1073/pnas.2106195118
- Primary Citation of Related Structures:
7LOA, 7LOB, 7LOC, 7LOD, 7LOE, 7LOF, 7LOG, 7LOJ, 7LX6, 7LX7, 7LX8, 7LX9, 7LXA - PubMed Abstract:
Protein flexibility remains a major challenge in library docking because of difficulties in sampling conformational ensembles with accurate probabilities. Here, we use the model cavity site of T4 lysozyme L99A to test flexible receptor docking with energy penalties from molecular dynamics (MD) simulations. Crystallography with larger and smaller ligands indicates that this cavity can adopt three major conformations: open, intermediate, and closed. Since smaller ligands typically bind better to the cavity site, we anticipate an energy penalty for the cavity opening. To estimate its magnitude, we calculate conformational preferences from MD simulations. We find that including a penalty term is essential for retrospective ligand enrichment; otherwise, high-energy states dominate the docking. We then prospectively docked a library of over 900,000 compounds for new molecules binding to each conformational state. Absent a penalty term, the open conformation dominated the docking results; inclusion of this term led to a balanced sampling of ligands against each state. High ranked molecules were experimentally tested by T m upshift and X-ray crystallography. From 33 selected molecules, we identified 18 ligands and determined 13 crystal structures. Most interesting were those bound to the open cavity, where the buried site opens to bulk solvent. Here, highly unusual ligands for this cavity had been predicted, including large ligands with polar tails; these were confirmed both by binding and by crystallography. In docking, incorporating protein flexibility with thermodynamic weightings may thus access new ligand chemotypes. The MD approach to accessing and, crucially, weighting such alternative states may find general applicability.
Organizational Affiliation:
Institute of General, Inorganic, and Theoretical Chemistry, Center for Molecular Biosciences Innsbruck, University of Innsbruck, 6020 Innsbruck, Austria.