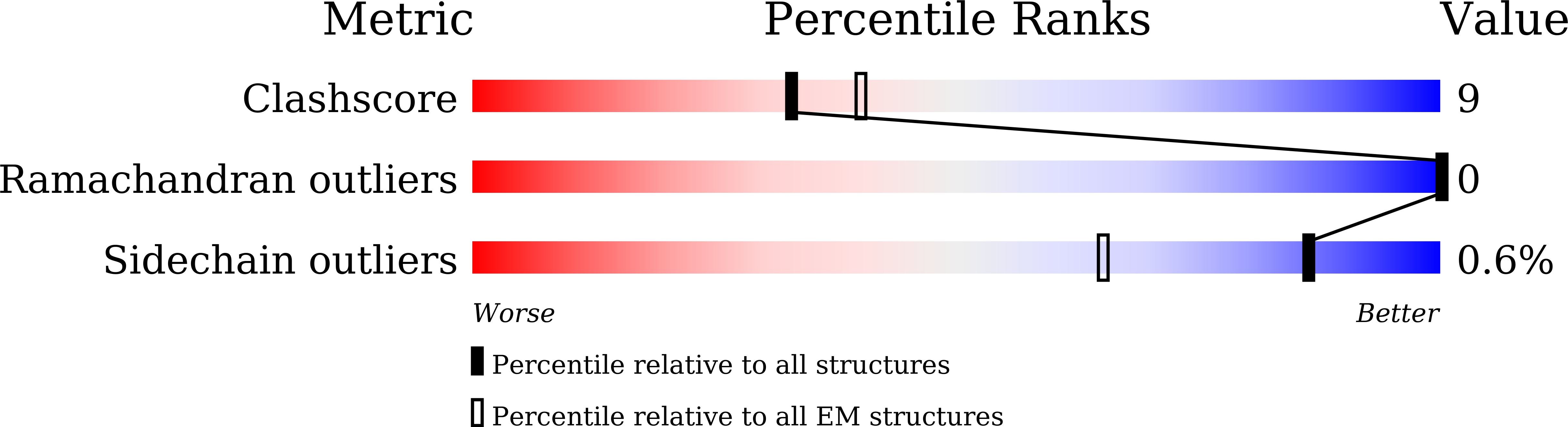
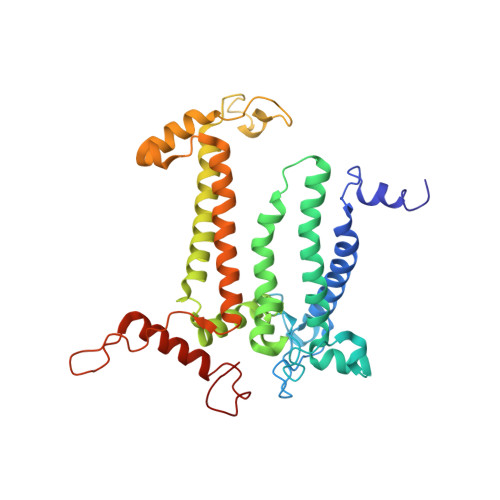
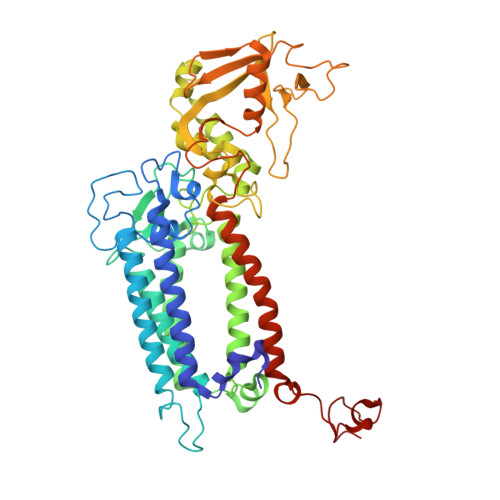
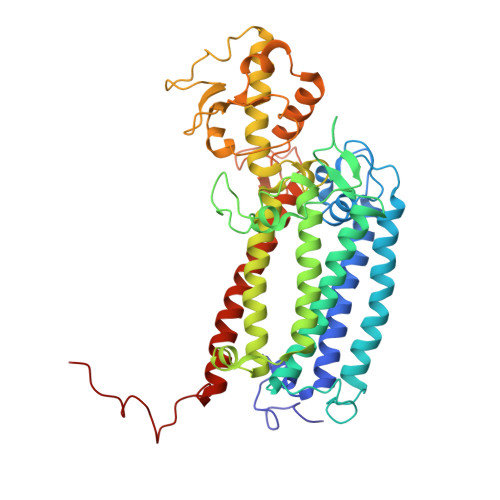
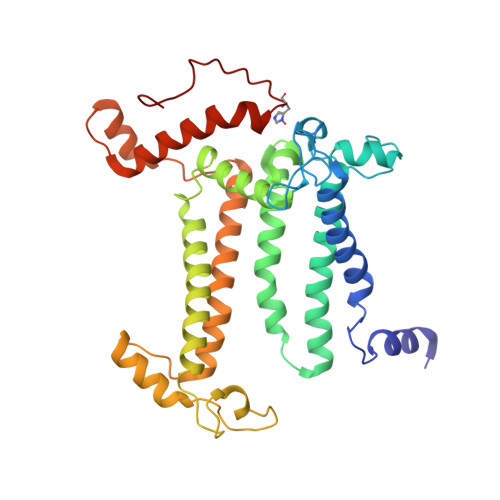
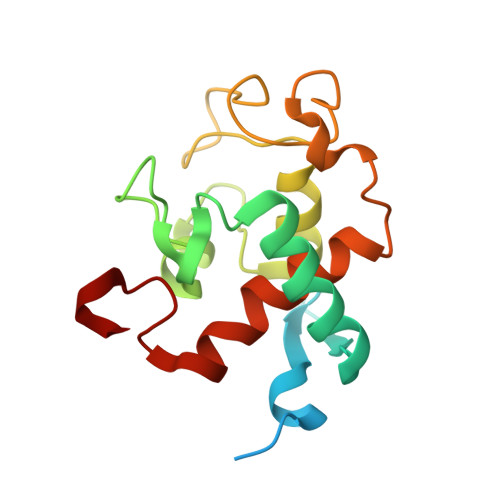
Cryo-EM structure of monomeric photosystem II at 2.78 angstrom resolution reveals factors important for the formation of dimer.
Yu, H., Hamaguchi, T., Nakajima, Y., Kato, K., Kawakami, K., Akita, F., Yonekura, K., Shen, J.R.(2021) Biochim Biophys Acta Bioenerg 1862: 148471-148471
- PubMed: 34216574
- DOI: https://doi.org/10.1016/j.bbabio.2021.148471
- Primary Citation of Related Structures:
7EDA - PubMed Abstract:
Photosystem II (PSII) functions mainly as a dimer to catalyze the light energy conversion and water oxidation reactions. However, monomeric PSII also exists and functions in vivo in some cases. The crystal structure of monomeric PSII has been solved at 3.6 Å resolution, but it is still not clear which factors contribute to the formation of the dimer. Here, we solved the structure of PSII monomer at a resolution of 2.78 Å using cryo-electron microscopy (cryo-EM). From our cryo-EM density map, we observed apparent differences in pigments and lipids in the monomer-monomer interface between the PSII monomer and dimer. One β-carotene and two sulfoquinovosyl diacylglycerol (SQDG) molecules are found in the monomer-monomer interface of the dimer structure but not in the present monomer structure, although some SQDG and other lipid molecules are found in the analogous region of the low-resolution crystal structure of the monomer, or cryo-EM structure of an apo-PSII monomer lacking the extrinsic proteins from Synechocystis sp. PCC 6803. In the current monomer structure, a large part of the PsbO subunit was also found to be disordered. These results indicate the importance of the β-carotene, SQDG and PsbO in formation of the PSII dimer.
Organizational Affiliation:
Research Institute for Interdisciplinary Science and Graduate School of Natural Science and Technology, Okayama University, 3-1-1 Tsushima Naka, Okayama 700-8530, Japan; Department of Picobiology, Graduate School of Life Science, University of Hyogo, Hyogo 678-1297, Japan.