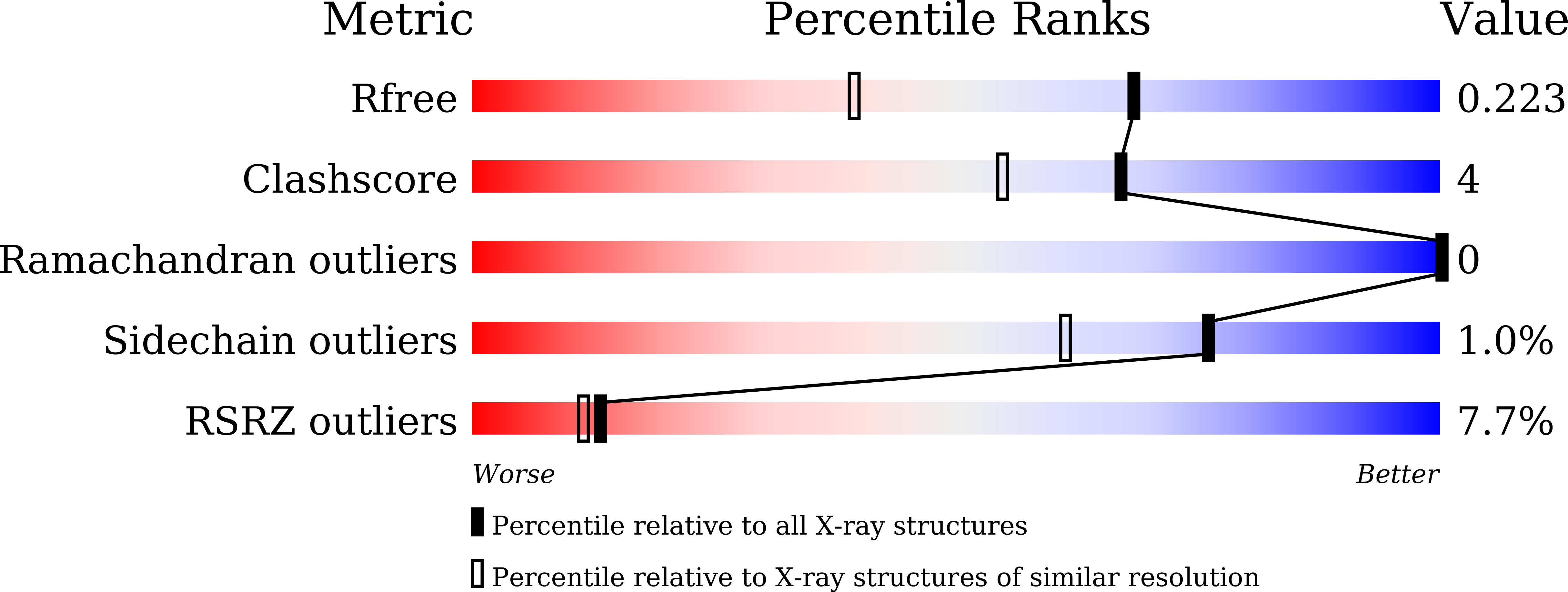
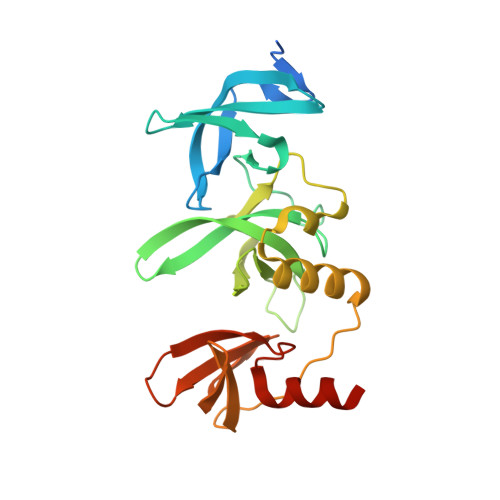
Structure-Guided Discovery of a Potent and Selective Cell-Active Inhibitor of SETDB1 Tudor Domain.
Guo, Y., Mao, X., Xiong, L., Xia, A., You, J., Lin, G., Wu, C., Huang, L., Wang, Y., Yang, S.(2021) Angew Chem Int Ed Engl 60: 8760-8765
- PubMed: 33511756
- DOI: https://doi.org/10.1002/anie.202017200
- Primary Citation of Related Structures:
7C9N, 7CAJ, 7CD9, 7CJT - PubMed Abstract:
SET domain bifurcated protein 1 (SETDB1) is a histone lysine methyltransferase that promotes the silencing of some tumour suppressor genes and is overexpressed in many cancers. SETDB1 contains a unique tandem tudor domain (TTD) that recognizes histone H3 sequences containing both methylated and acetylated lysines. Beginning with the identification of a hit compound (Cpd1), we discovered the first potent and selective small molecule SETDB1-TTD inhibitor (R,R)-59 through stepwise structure-guided optimization. (R,R)-59 showed a K D value of 0.088±0.045 μM in the ITC assay. The high potency of (R,R)-59 was well explained by the cocrystal structure of the (R,R)-59-TTD complex. (R,R)-59 is an endogenous binder competitive inhibitor. Evidence has also demonstrated its cellular target engagement. Interestingly, the enantiomer (S,S)-59 did not show activity in all the assays, highlighting the potential of (R,R)-59 as a tool compound in exploring the biological functions of SETDB1-TTD.
Organizational Affiliation:
State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041, P. R. China.