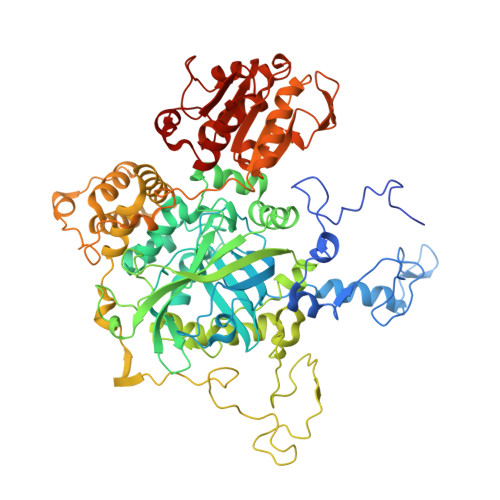
Serendipitous crystallization of E. coli HPII catalase, a sequel to "the tale usually not told".
Grzechowiak, M., Sekula, B., Jaskolski, M., Ruszkowski, M.(2021) Acta Biochim Pol 68: 29-31
- PubMed: 33485289
- DOI: https://doi.org/10.18388/abp.2020_5501
- Primary Citation of Related Structures:
6ZTV, 6ZTW, 6ZTX - PubMed Abstract:
Protein crystallographers are well aware of the trap of crystallizing E. coli proteins instead of the macromolecule of interest if heterologous recombinant protein expression in E. coli was part of the experimental pipeline. Among the well-known culprits are YodA metal-binding lipocalin (25 kDa) and YadF carbonic anhydrase (a tetramer of 25 kDa subunits). We report a novel crystal form of another such culprit, E. coli HPII catalase, which is a tetrameric protein of ~340 kDa molecular weight. HPII is likely to contaminate recombinant protein samples, co-purify, and then co-crystallize with the target proteins, especially if their masses in size exclusion chromatography are ~300-400 kDa. What makes this case more interesting but also parlous, is the fact that HPII can crystallize from very low concentrations, even well below 1 mg/mL.
Organizational Affiliation:
Institute of Bioorganic Chemistry, Polish Academy of Sciences, Poznań, Poland.