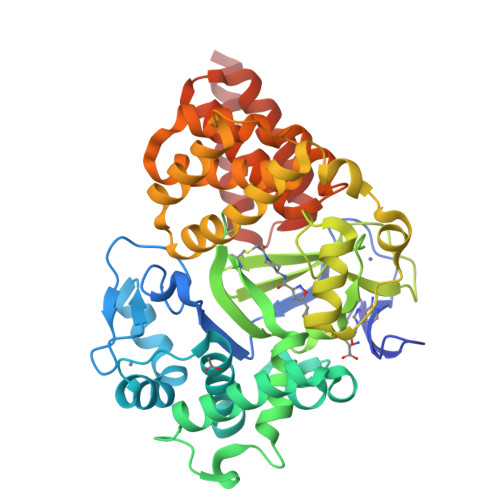
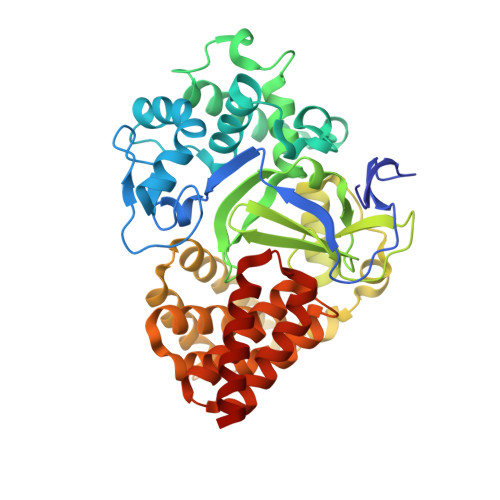
Discovery of the 4-aminopiperidine-based compound EM127 for the site-specific covalent inhibition of SMYD3
Parenti, M.D., Naldi, M., Manoni, E., Fabini, E., Cederfelt, D., Talibov, V.O., Gressani, V., Guven, U., Grossi, V., Fasano, C., Sanese, P., De Marco, K., Shtil, A.A., Kurkin, A.V., Altieri, A., Danielson, U.H., Caretti, G., Simone, C., Varchi, G., Bartolini, M., Del Rio, A.(2022) Eur J Med Chem : 114683