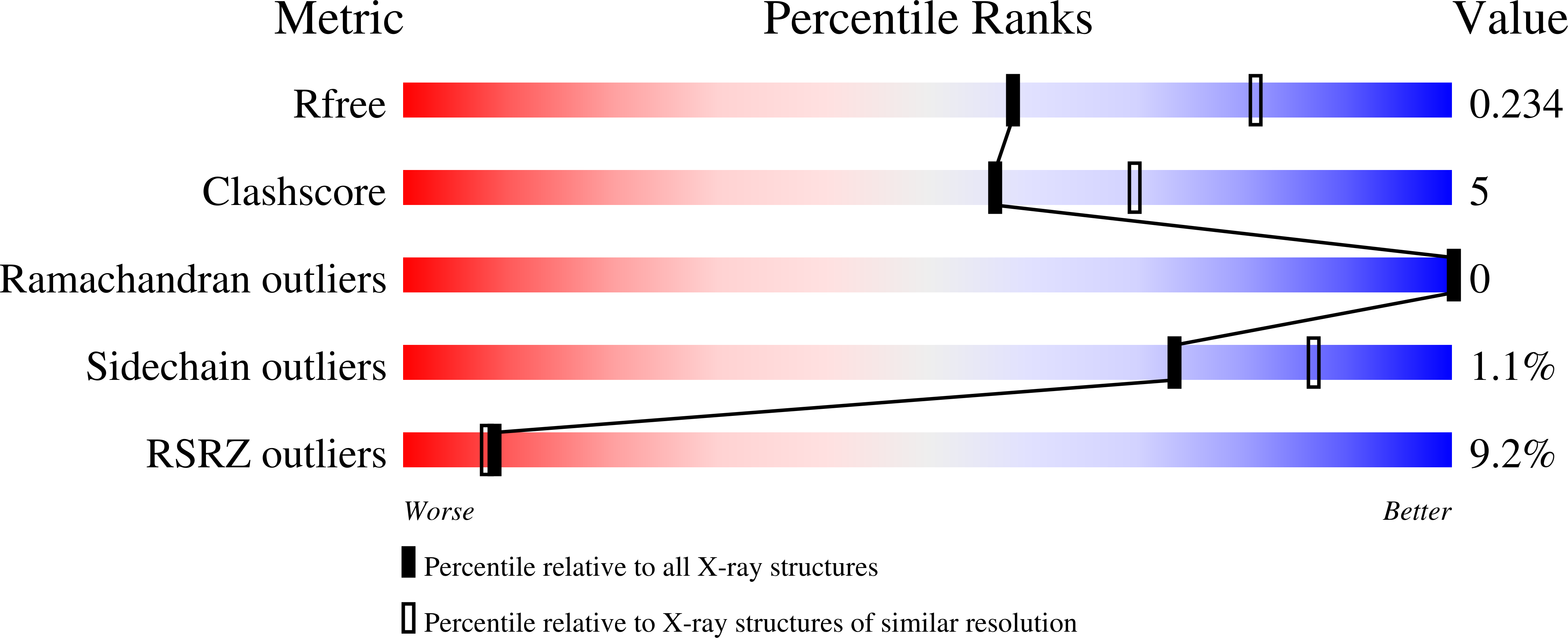
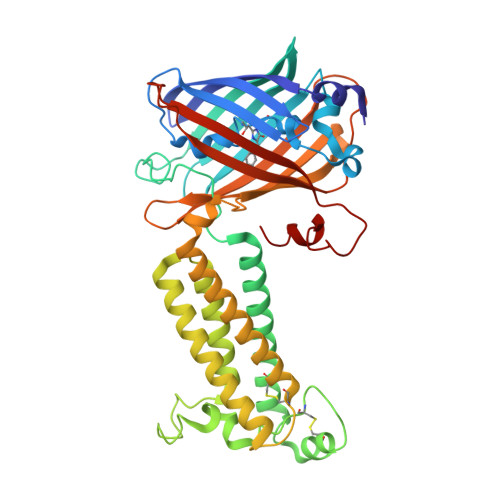
Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation.
Liu, S., Li, S., Shen, G., Sukumar, N., Krezel, A.M., Li, W.(2021) Science 371
- PubMed: 33154105
- DOI: https://doi.org/10.1126/science.abc5667
- Primary Citation of Related Structures:
6WV3, 6WV4, 6WV5, 6WV6, 6WV7, 6WV8, 6WV9, 6WVA, 6WVB, 6WVH, 6WVI - PubMed Abstract:
Vitamin K antagonists are widely used anticoagulants that target vitamin K epoxide reductases (VKOR), a family of integral membrane enzymes. To elucidate their catalytic cycle and inhibitory mechanism, we report 11 x-ray crystal structures of human VKOR and pufferfish VKOR-like, with substrates and antagonists in different redox states. Substrates entering the active site in a partially oxidized state form cysteine adducts that induce an open-to-closed conformational change, triggering reduction. Binding and catalysis are facilitated by hydrogen-bonding interactions in a hydrophobic pocket. The antagonists bind specifically to the same hydrogen-bonding residues and induce a similar closed conformation. Thus, vitamin K antagonists act through mimicking the key interactions and conformational changes required for the VKOR catalytic cycle.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, MO 63110, USA.