Structures of the human mitochondrial ribosome bound to EF-G1 reveal distinct features of mitochondrial translation elongation.
Koripella, R.K., Sharma, M.R., Bhargava, K., Datta, P.P., Kaushal, P.S., Keshavan, P., Spremulli, L.L., Banavali, N.K., Agrawal, R.K.(2020) Nat Commun 11: 3830-3830
- PubMed: 32737313
- DOI: https://doi.org/10.1038/s41467-020-17715-2
- Primary Citation of Related Structures:
6VLZ, 6VMI - PubMed Abstract:
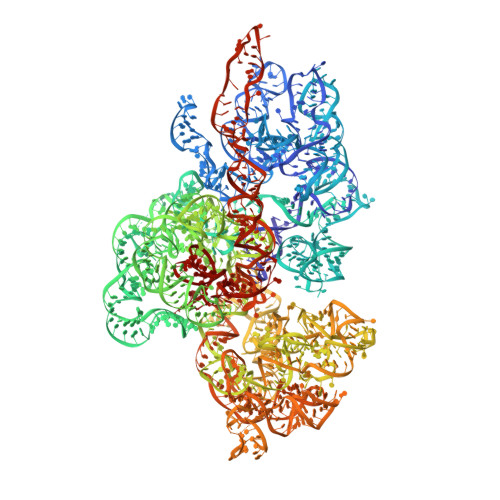
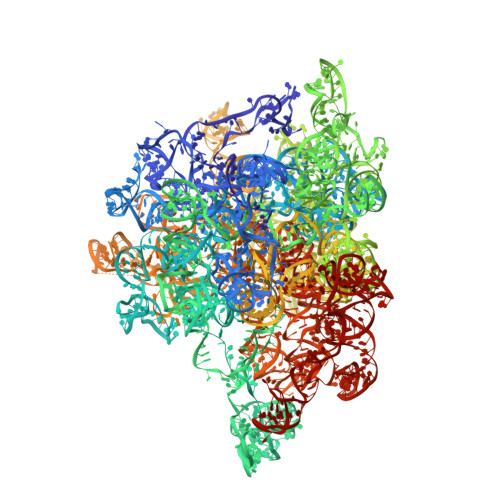
The mammalian mitochondrial ribosome (mitoribosome) and its associated translational factors have evolved to accommodate greater participation of proteins in mitochondrial translation. Here we present the 2.68-3.96 Å cryo-EM structures of the human 55S mitoribosome in complex with the human mitochondrial elongation factor G1 (EF-G1 mt ) in three distinct conformational states, including an intermediate state and a post-translocational state. These structures reveal the role of several mitochondria-specific (mito-specific) mitoribosomal proteins (MRPs) and a mito-specific segment of EF-G1 mt in mitochondrial tRNA (tRNA mt ) translocation. In particular, the mito-specific C-terminal extension in EF-G1 mt is directly involved in translocation of the acceptor arm of the A-site tRNA mt . In addition to the ratchet-like and independent head-swiveling motions exhibited by the small mitoribosomal subunit, we discover significant conformational changes in MRP mL45 at the nascent polypeptide-exit site within the large mitoribosomal subunit that could be critical for tethering of the elongating mitoribosome onto the inner-mitochondrial membrane.
Organizational Affiliation:
Division of Translational Medicine, Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, 12201, USA.