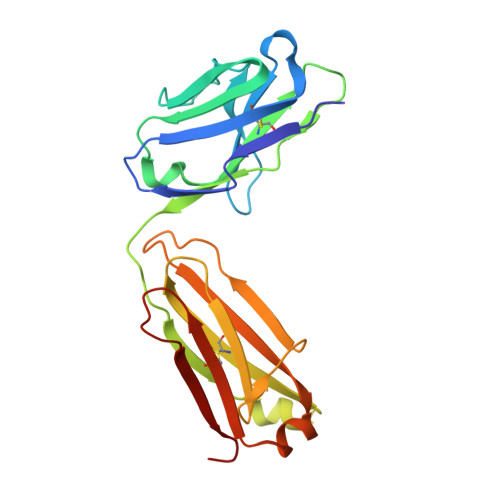
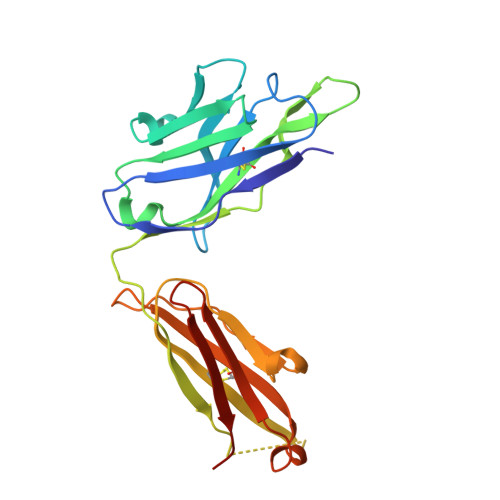
An MPER antibody neutralizes HIV-1 using germline features shared among donors.
Zhang, L., Irimia, A., He, L., Landais, E., Rantalainen, K., Leaman, D.P., Vollbrecht, T., Stano, A., Sands, D.I., Kim, A.S., Poignard, P., Burton, D.R., Murrell, B., Ward, A.B., Zhu, J., Wilson, I.A., Zwick, M.B.(2019) Nat Commun 10: 5389-5389
- PubMed: 31772165
- DOI: https://doi.org/10.1038/s41467-019-12973-1
- Primary Citation of Related Structures:
6O3D, 6O3G, 6O3J, 6O3K, 6O3L, 6O3U, 6O41, 6O42 - PubMed Abstract:
The membrane-proximal external region (MPER) of HIV-1 envelope glycoprotein (Env) can be targeted by neutralizing antibodies of exceptional breadth. MPER antibodies usually have long, hydrophobic CDRH3s, lack activity as inferred germline precursors, are often from the minor IgG3 subclass, and some are polyreactive, such as 4E10. Here we describe an MPER broadly neutralizing antibody from the major IgG1 subclass, PGZL1, which shares germline V/D-region genes with 4E10, has a shorter CDRH3, and is less polyreactive. A recombinant sublineage variant pan-neutralizes a 130-isolate panel at 1.4 μg/ml (IC 50 ). Notably, a germline revertant with mature CDR3s neutralizes 12% of viruses and still binds MPER after DJ reversion. Crystal structures of lipid-bound PGZL1 variants and cryo-EM reconstruction of an Env-PGZL1 complex reveal how these antibodies recognize MPER and viral membrane. Discovery of common genetic and structural elements among MPER antibodies from different patients suggests that such antibodies could be elicited using carefully designed immunogens.
Organizational Affiliation:
Department of Immunology and Microbiology, The Scripps Research Institute, La Jolla, California, 92037, USA.