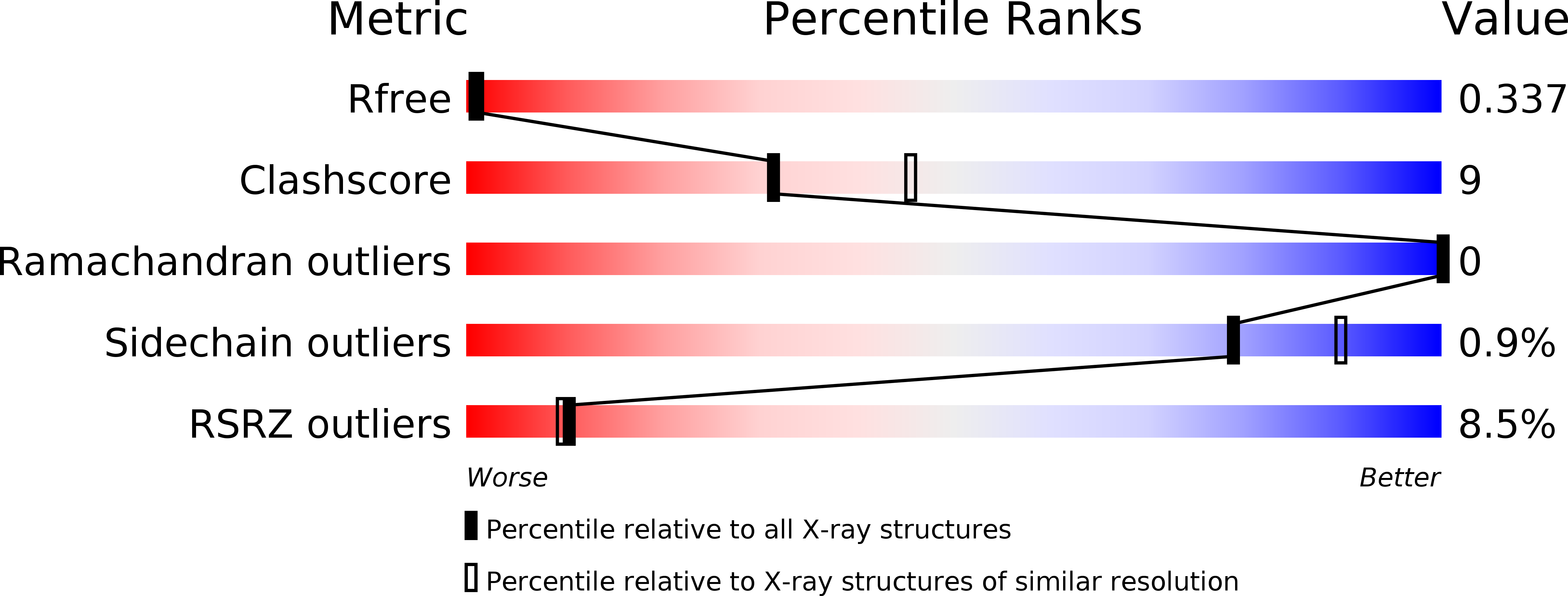
Hitting a Moving Target: Simulation and Crystallography Study of ATAD2 Bromodomain Blockers.
Dolbois, A., Batiste, L., Wiedmer, L., Dong, J., Brutsch, M., Huang, D., Deerain, N.M., Spiliotopoulos, D., Cheng-Sanchez, I., Laul, E., Nevado, C., Sledz, P., Caflisch, A.(2020) ACS Med Chem Lett 11: 1573-1580
- PubMed: 32832026
- DOI: https://doi.org/10.1021/acsmedchemlett.0c00080
- Primary Citation of Related Structures:
5F36, 6EPJ, 6EPT, 6EPU, 6EPV, 6EPX, 6HI3, 6HI4, 6HI5, 6HI6, 6HI7, 6HI8, 6HIA, 6HIB, 6HIC, 6HID, 6HIE - PubMed Abstract:
Small molecule ligand binding to the ATAD2 bromodomain is investigated here through the synergistic combination of molecular dynamics and protein crystallography. A previously unexplored conformation of the binding pocket upon rearrangement of the gatekeeper residue Ile1074 has been found. Further, our investigations reveal how minor structural differences in the ligands result in binding with different plasticity of the ZA loop for this difficult-to-drug bromodomain.
Organizational Affiliation:
Department of Chemistry and Department of Biochemistry, University of Zurich, Winterthurerstrasse 190, 8057 Zurich, Switzerland.