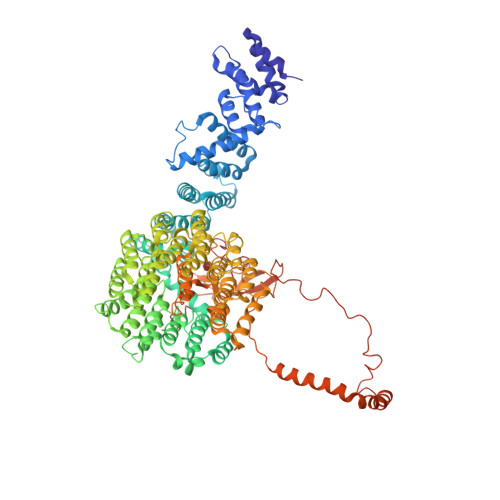
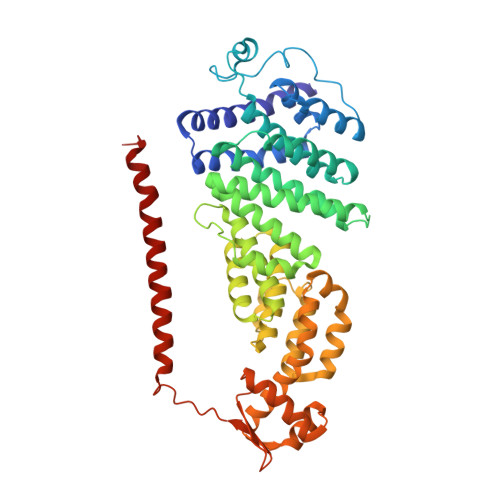
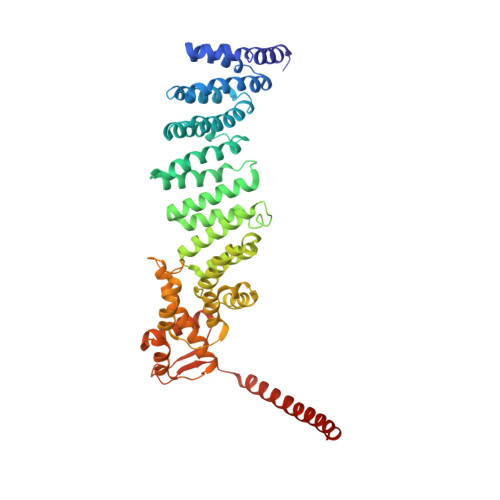
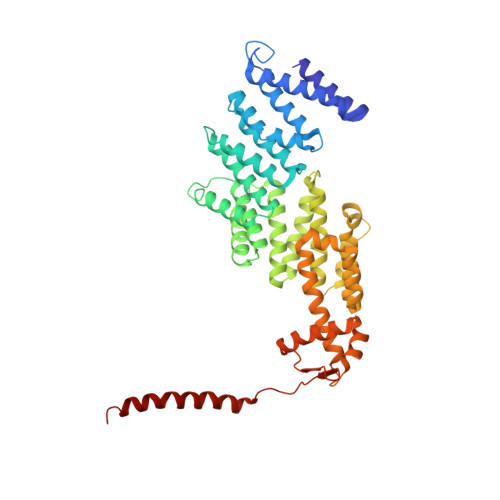
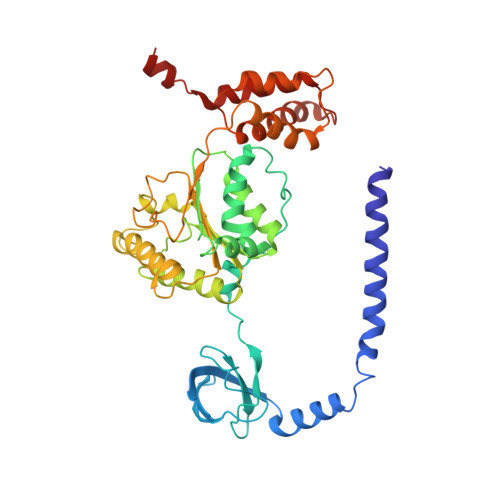
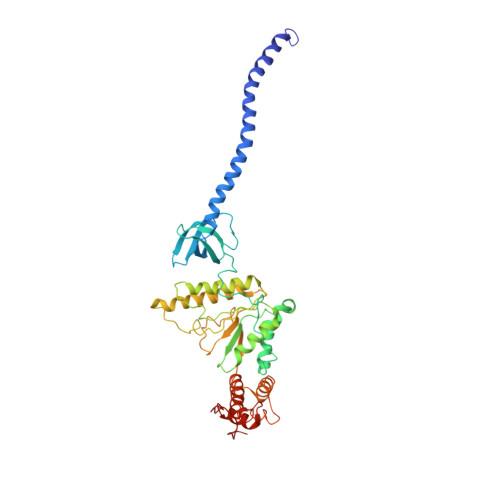
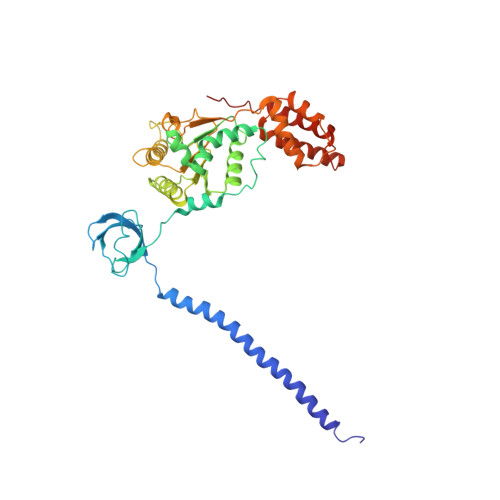
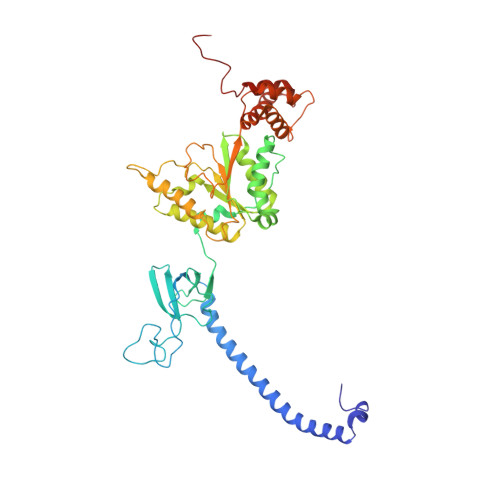
Expanded Coverage of the 26S Proteasome Conformational Landscape Reveals Mechanisms of Peptidase Gating.
Eisele, M.R., Reed, R.G., Rudack, T., Schweitzer, A., Beck, F., Nagy, I., Pfeifer, G., Plitzko, J.M., Baumeister, W., Tomko Jr., R.J., Sakata, E.(2018) Cell Rep 24: 1301-1315.e5
- PubMed: 30067984
- DOI: https://doi.org/10.1016/j.celrep.2018.07.004
- Primary Citation of Related Structures:
6FVT, 6FVU, 6FVV, 6FVW, 6FVX, 6FVY - PubMed Abstract:
The proteasome is the central protease for intracellular protein breakdown. Coordinated binding and hydrolysis of ATP by the six proteasomal ATPase subunits induces conformational changes that drive the unfolding and translocation of substrates into the proteolytic 20S core particle for degradation. Here, we combine genetic and biochemical approaches with cryo-electron microscopy and integrative modeling to dissect the relationship between individual nucleotide binding events and proteasome conformational dynamics. We demonstrate unique impacts of ATP binding by individual ATPases on the proteasome conformational distribution and report two conformational states of the proteasome suggestive of a rotary ATP hydrolysis mechanism. These structures, coupled with functional analyses, reveal key roles for the ATPases Rpt1 and Rpt6 in gating substrate entry into the core particle. This deepened knowledge of proteasome conformational dynamics reveals key elements of intersubunit communication within the proteasome and clarifies the regulation of substrate entry into the proteolytic chamber.
Organizational Affiliation:
Department of Molecular Structural Biology, Max Planck Institute of Biochemistry, 82152 Martinsried, Germany.