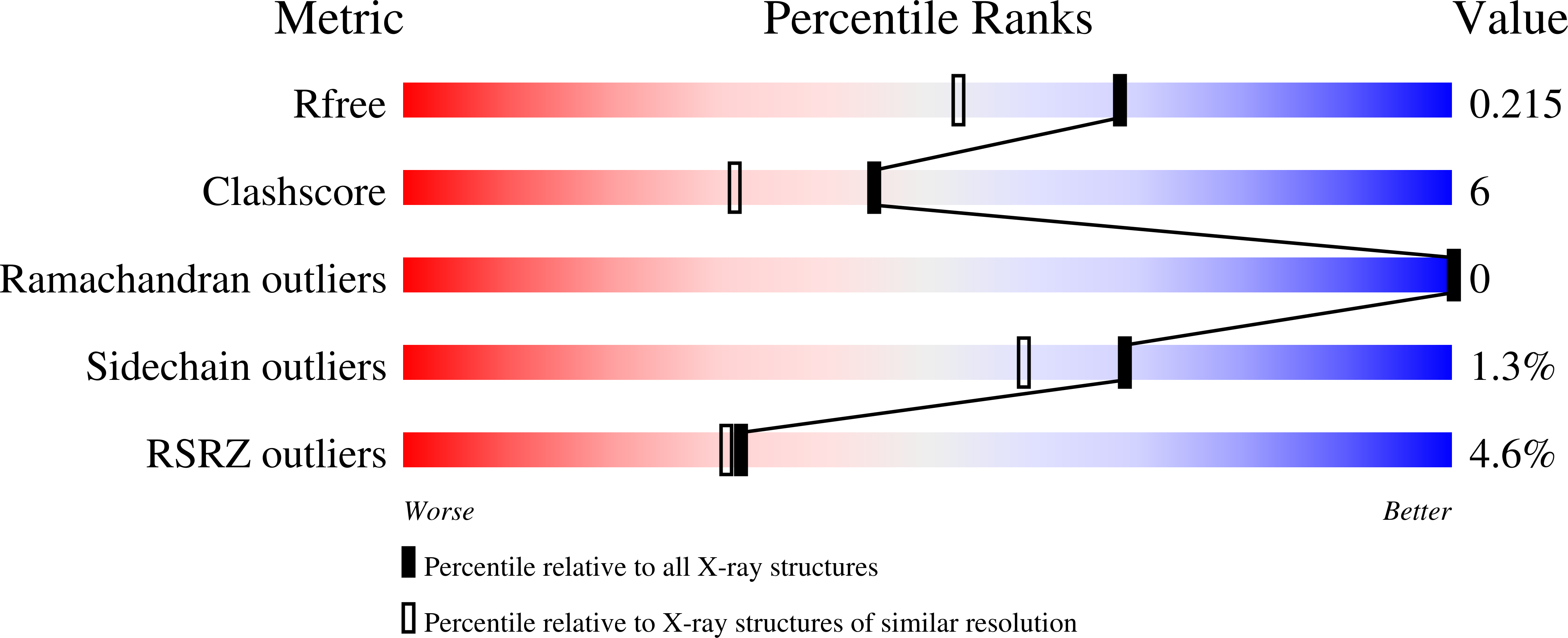
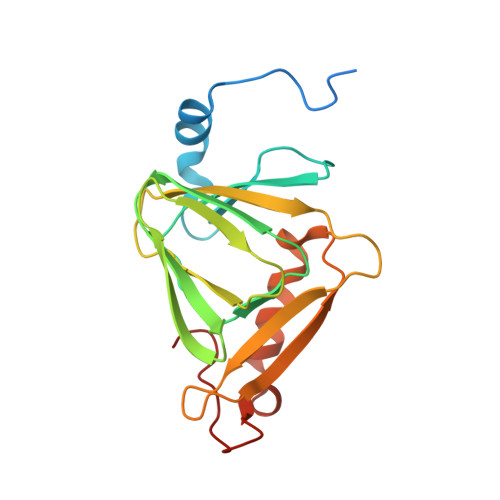
Adapting to oxygen: 3-Hydroxyanthrinilate 3,4-dioxygenase employs loop dynamics to accommodate two substrates with disparate polarities.
Yang, Y., Liu, F., Liu, A.(2018) J Biol Chem 293: 10415-10424
- PubMed: 29784877
- DOI: https://doi.org/10.1074/jbc.RA118.002698
- Primary Citation of Related Structures:
6BVP, 6BVQ, 6BVR, 6BVS, 6CD3, 6D60, 6D61, 6D62 - PubMed Abstract:
3-Hydroxyanthranilate 3,4-dioxygenase (HAO) is an iron-dependent protein that activates O 2 and inserts both oxygen atoms into 3-hydroxyanthranilate (3-HAA). An intriguing question is how HAO can rapidly bind O 2 , even though local O 2 concentrations and diffusion rates are relatively low. Here, a close inspection of the HAO structures revealed that substrate- and inhibitor-bound structures exhibit a closed conformation with three hydrophobic loop regions moving toward the catalytic iron center, whereas the ligand-free structure is open. We hypothesized that these loop movements enhance O 2 binding to the binary complex of HAO and 3-HAA. We found that the carboxyl end of 3-HAA triggers changes in two loop regions and that the third loop movement appears to be driven by an H-bond interaction between Asn 27 and Ile 142 Mutational analyses revealed that N27A, I142A, and I142P variants cannot form a closed conformation, and steady-state kinetic assays indicated that these variants have a substantially higher K m for O 2 than WT HAO. This observation suggested enhanced hydrophobicity at the iron center resulting from the concerted loop movements after the binding of the primary substrate, which is hydrophilic. Given that O 2 is nonpolar, the increased hydrophobicity at the iron center of the binary complex appears to be essential for rapid O 2 binding and activation, explaining the reason for the 3-HAA-induced loop movements. Because substrate binding-induced open-to-closed conformational changes are common, the results reported here may help further our understanding of how oxygen is enriched in nonheme iron-dependent dioxygenases.
Organizational Affiliation:
From the Department of Chemistry, University of Texas at San Antonio, San Antonio, Texas 78249 and.