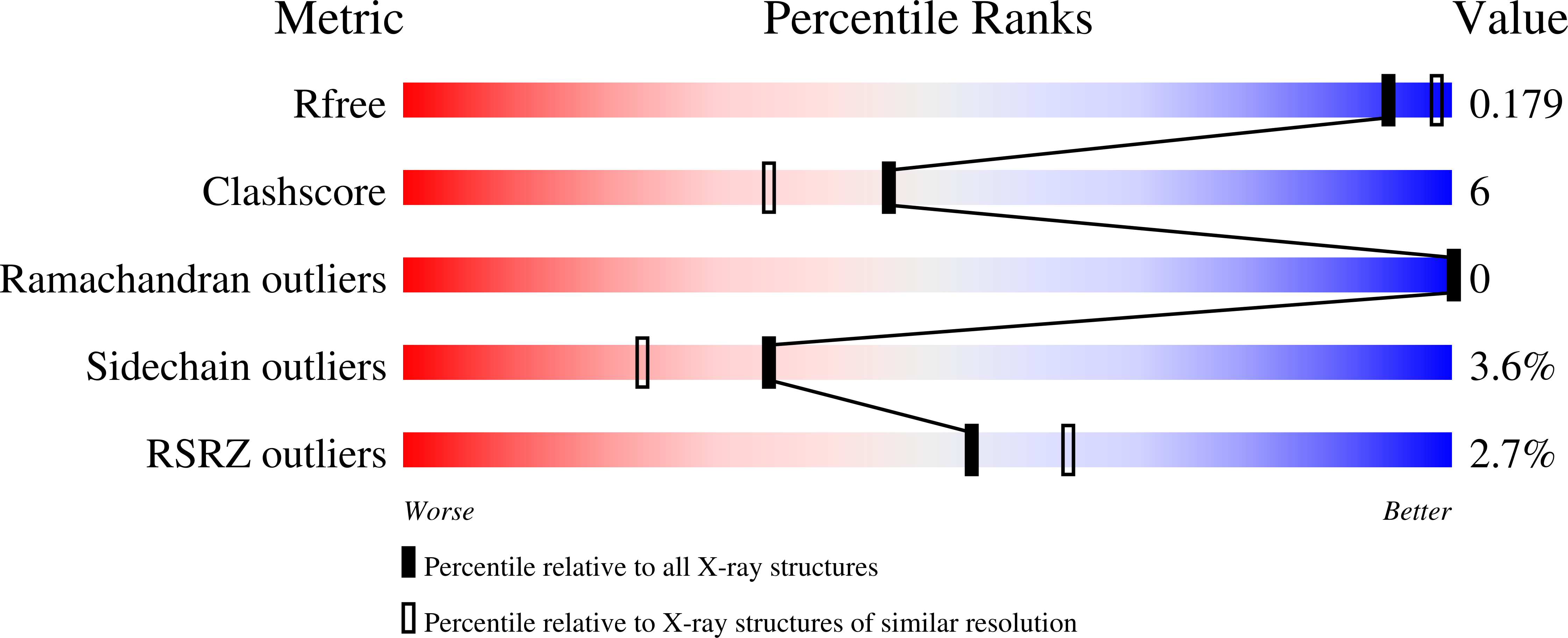
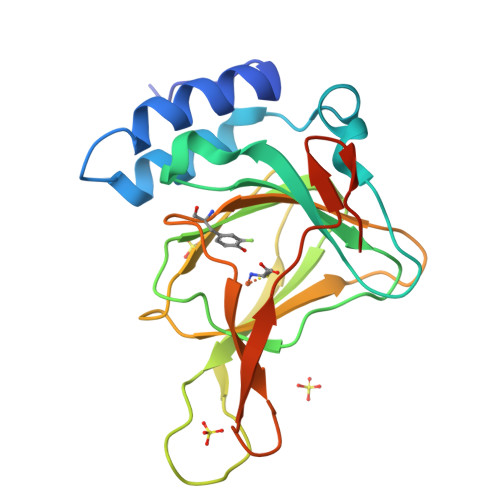
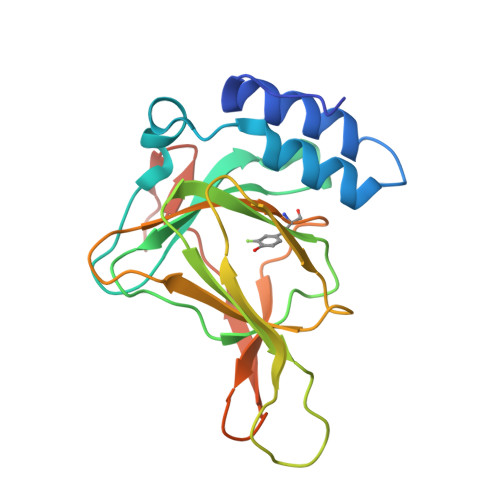
Cleavage of a carbon-fluorine bond by an engineered cysteine dioxygenase.
Li, J., Griffith, W.P., Davis, I., Shin, I., Wang, J., Li, F., Wang, Y., Wherritt, D.J., Liu, A.(2018) Nat Chem Biol 14: 853-860
- PubMed: 29942080
- DOI: https://doi.org/10.1038/s41589-018-0085-5
- Primary Citation of Related Structures:
6BGF, 6BGM, 6BPS, 6BPT, 6BPU, 6BPV, 6BPW, 6BPX, 6CDH, 6CDN - PubMed Abstract:
Cysteine dioxygenase (CDO) plays an essential role in sulfur metabolism by regulating homeostatic levels of cysteine. Human CDO contains a post-translationally generated Cys93-Tyr157 cross-linked cofactor. Here, we investigated this Cys-Tyr cross-linking by incorporating unnatural tyrosines in place of Tyr157 via a genetic method. The catalytically active variants were obtained with a thioether bond between Cys93 and the halogen-substituted Tyr157, and we determined the crystal structures of both wild-type and engineered CDO variants in the purely uncross-linked form and with a mature cofactor. Along with mass spectrometry and 19 F NMR, these data indicated that the enzyme could catalyze oxidative C-F or C-Cl bond cleavage, resulting in a substantial conformational change of both Cys93 and Tyr157 during cofactor assembly. These findings provide insights into the mechanism of Cys-Tyr cofactor biogenesis and may aid the development of bioinspired aromatic carbon-halogen bond activation.
Organizational Affiliation:
Department of Chemistry, University of Texas at San Antonio, San Antonio, TX, USA.