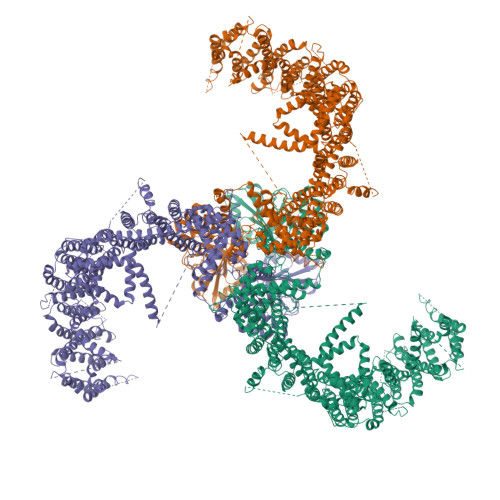
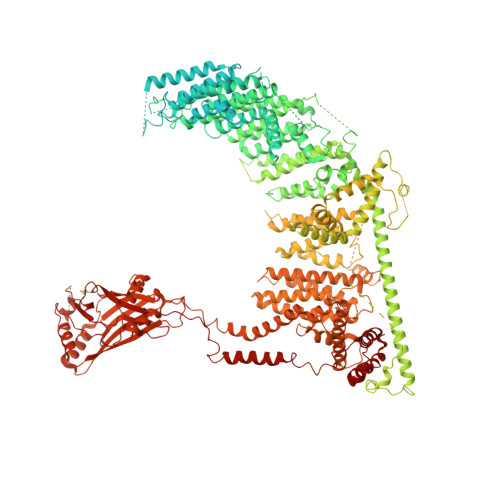
Structure and mechanogating mechanism of the Piezo1 channel.
Zhao, Q., Zhou, H., Chi, S., Wang, Y., Wang, J., Geng, J., Wu, K., Liu, W., Zhang, T., Dong, M.Q., Wang, J., Li, X., Xiao, B.(2018) Nature 554: 487-492
- PubMed: 29469092
- DOI: https://doi.org/10.1038/nature25743
- Primary Citation of Related Structures:
5Z10 - PubMed Abstract:
The mechanosensitive Piezo channels function as key eukaryotic mechanotransducers. However, their structures and mechanogating mechanisms remain unknown. Here we determine the three-bladed, propeller-like electron cryo-microscopy structure of mouse Piezo1 and functionally reveal its mechanotransduction components. Despite the lack of sequence repetition, we identify nine repetitive units consisting of four transmembrane helices each-which we term transmembrane helical units (THUs)-which assemble into a highly curved blade-like structure. The last transmembrane helix encloses a hydrophobic pore, followed by three intracellular fenestration sites and side portals that contain pore-property-determining residues. The central region forms a 90 Å-long intracellular beam-like structure, which undergoes a lever-like motion to connect THUs to the pore via the interfaces of the C-terminal domain, the anchor-resembling domain and the outer helix. Deleting extracellular loops in the distal THUs or mutating single residues in the beam impairs the mechanical activation of Piezo1. Overall, Piezo1 possesses a unique 38-transmembrane-helix topology and designated mechanotransduction components, which enable a lever-like mechanogating mechanism.
Organizational Affiliation:
School of Pharmaceutical Sciences or Life Sciences, Tsinghua University, Beijing 100084, China.