Introducing site-specific cysteines into nanobodies for mercury labelling allows de novo phasing of their crystal structures.
Hansen, S.B., Laursen, N.S., Andersen, G.R., Andersen, K.R.(2017) Acta Crystallogr D Struct Biol 73: 804-813
- PubMed: 28994409
- DOI: https://doi.org/10.1107/S2059798317013171
- Primary Citation of Related Structures:
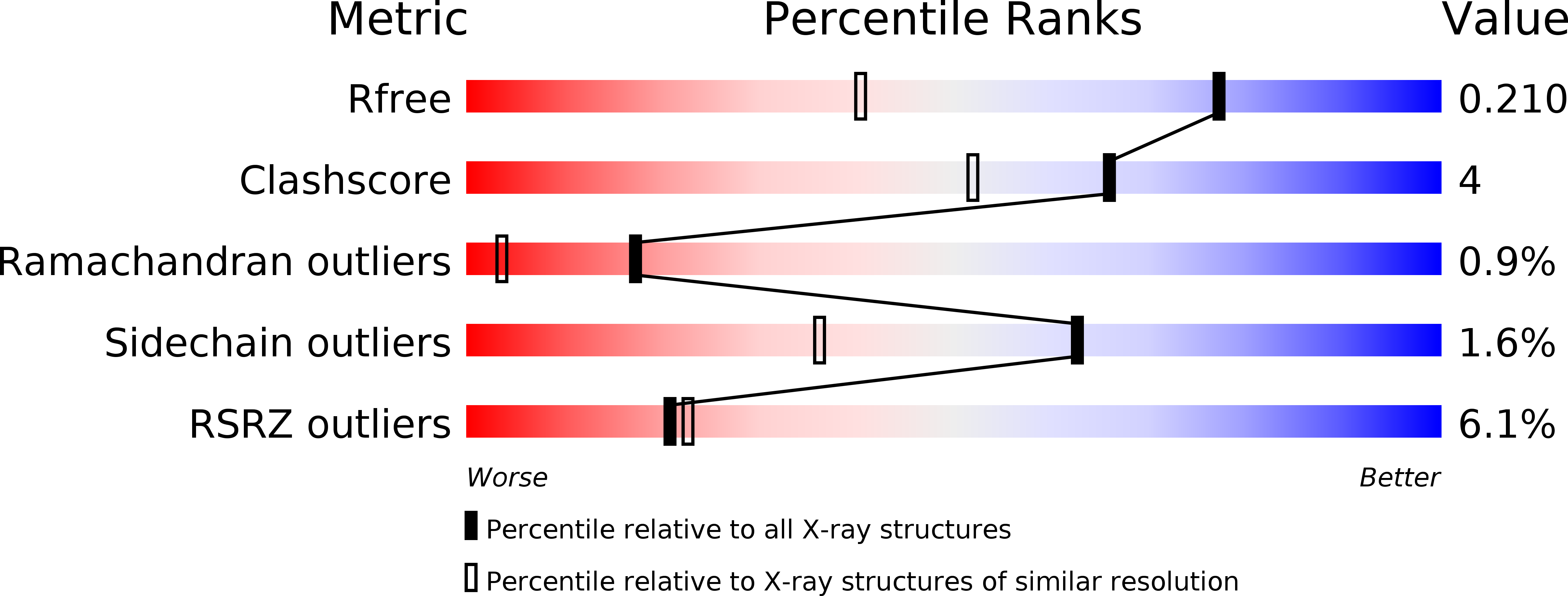
5NLU, 5NLW, 5NM0, 5NML - PubMed Abstract:
The generation of high-quality protein crystals and the loss of phase information during an X-ray crystallography diffraction experiment represent the major bottlenecks in the determination of novel protein structures. A generic method for introducing Hg atoms into any crystal independent of the presence of free cysteines in the target protein could considerably facilitate the process of obtaining unbiased experimental phases. Nanobodies (single-domain antibodies) have recently been shown to promote the crystallization and structure determination of flexible proteins and complexes. To extend the usability of nanobodies for crystallographic work, variants of the Nb36 nanobody with a single free cysteine at one of four framework-residue positions were developed. These cysteines could be labelled with fluorophores or Hg. For one cysteine variant (Nb36-C85) two nanobody structures were experimentally phased using single-wavelength anomalous dispersion (SAD) and single isomorphous replacement with anomalous signal (SIRAS), taking advantage of radiation-induced changes in Cys-Hg bonding. Importantly, Hg labelling influenced neither the interaction of Nb36 with its antigen complement C5 nor its structure. The results suggest that Cys-Hg-labelled nanobodies may become efficient tools for obtaining de novo phase information during the structure determination of nanobody-protein complexes.
Organizational Affiliation:
Department of Molecular Biology and Genetics, Aarhus University, Gustav Wieds Vej 10C, 8000 Aarhus, Denmark.