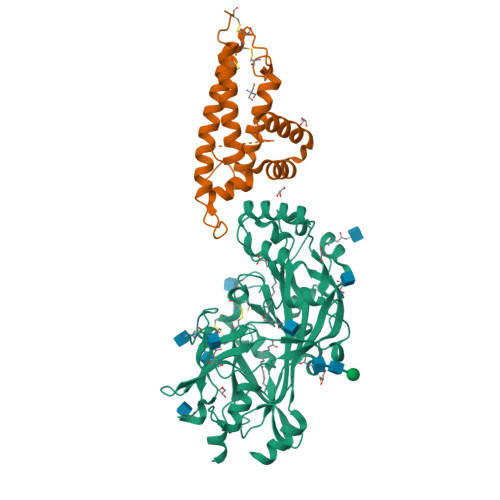
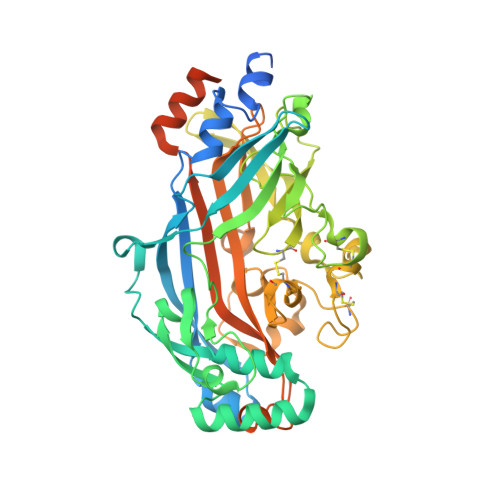
The structural basis for CD36 binding by the malaria parasite.
Hsieh, F.L., Turner, L., Bolla, J.R., Robinson, C.V., Lavstsen, T., Higgins, M.K.(2016) Nat Commun 7: 12837-12837
- PubMed: 27667267
- DOI: https://doi.org/10.1038/ncomms12837
- Primary Citation of Related Structures:
5LGD - PubMed Abstract:
CD36 is a scavenger receptor involved in fatty acid metabolism, innate immunity and angiogenesis. It interacts with lipoprotein particles and facilitates uptake of long chain fatty acids. It is also the most common target of the PfEMP1 proteins of the malaria parasite, Plasmodium falciparum, tethering parasite-infected erythrocytes to endothelial receptors. This prevents their destruction by splenic clearance and allows increased parasitaemia. Here we describe the structure of CD36 in complex with long chain fatty acids and a CD36-binding PfEMP1 protein domain. A conserved hydrophobic pocket allows the hugely diverse PfEMP1 protein family to bind to a conserved phenylalanine residue at the membrane distal tip of CD36. This phenylalanine is also required for CD36 to interact with lipoprotein particles. By targeting a site on CD36 that is required for its physiological function, PfEMP1 proteins maintain the ability to tether to the endothelium and avoid splenic clearance.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, UK.