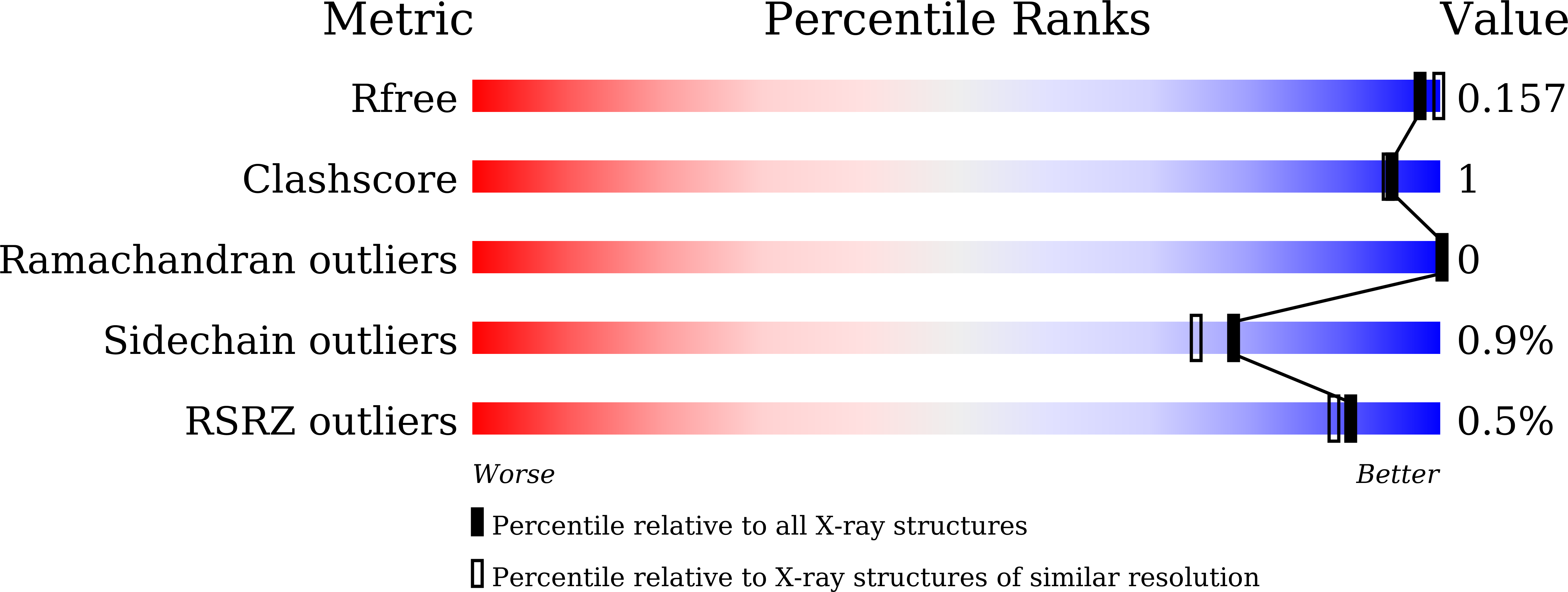
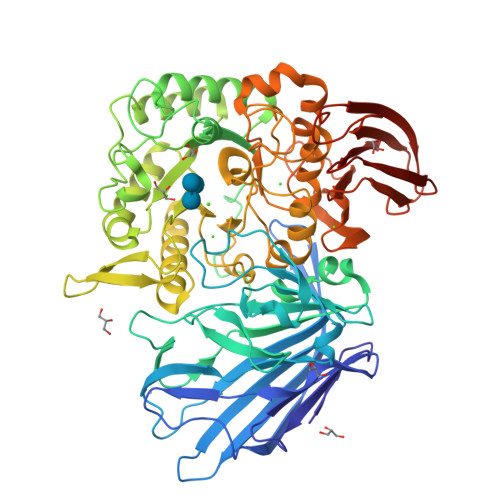
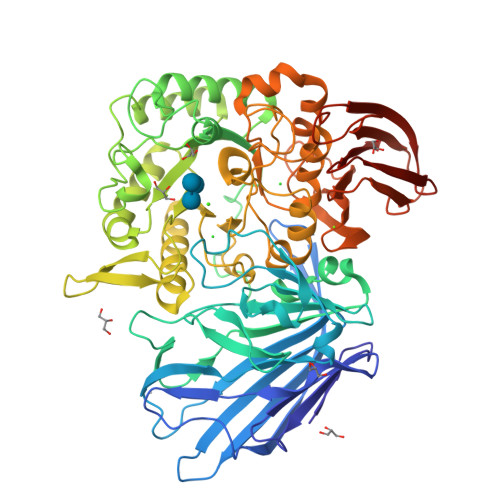
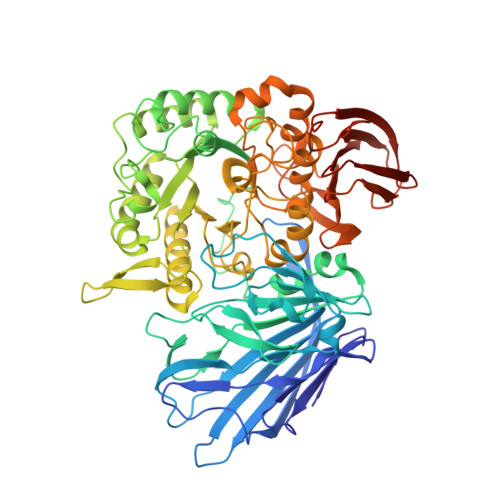
Structures of PspAG97A alpha-glucoside hydrolase reveal a novel mechanism for chloride induced activation.
He, C., Li, J., Li, W., Xue, Y., Fang, Z., Fang, W., Zhang, X., Wang, X., Xiao, Y.(2016) J Struct Biol 196: 426-436
- PubMed: 27645700
- DOI: https://doi.org/10.1016/j.jsb.2016.09.009
- Primary Citation of Related Structures:
5HQ4, 5HQA, 5HQB, 5HQC - PubMed Abstract:
Here we report the first crystal structure of a secretory α-glucoside hydrolase isolated from Pseudoalteromonas sp. K8, PspAG97A, which belongs to glycoside hydrolase family 97 and exhibits halophilic property. PspAG97A lacks an acidic surface, that is considered essential for protein stability at high salinity. Interestingly, PspAG97A unusually contains a chloride ion coordinated by the guanidinium group of Arg171 and the main chain amide groups of Tyr172 and Glu173 at the active site. The structures of PspAG97A complexed with acarbose and panose demonstrate that residues Glu173, Arg171 and Asn170 for subsite +1 decide the substrate specificity of the enzyme for the α-1,6-glucosidic linkage. Structural alterations observed in the R171K variant and enzyme kinetic experiments focusing on chloride assisted activation suggest that the active site chloride serves to properly orient Glu173, Arg171 and Asn170 to facilitate substrate recognition. Furthermore, the chloride assists the binding of Glu173 to the conserved calcium ion and plays an essential role in properly positioning the base catalyst Glu456. In sum, our results provide valuable insight into the structural basis of protein halophilicity.
Organizational Affiliation:
School of Life Sciences, Anhui University, Hefei, Anhui 230601, China; Anhui Provincial Engineering Technology Research Center of Microorganisms and Biocatalysis, Hefei, Anhui 230601, China.