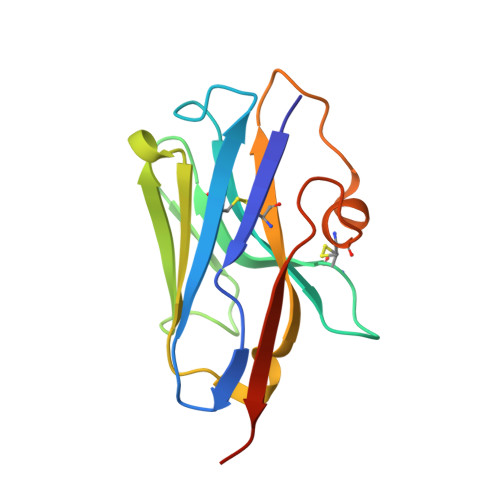
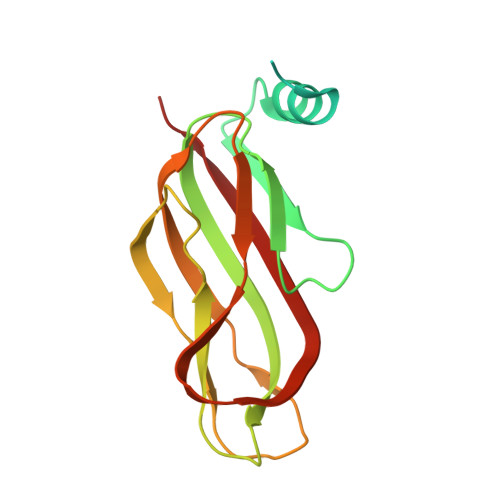
The Atomic Structure of the Phage Tuc2009 Baseplate Tripod Suggests that Host Recognition Involves Two Different Carbohydrate Binding Modules.
Legrand, P., Collins, B., Blangy, S., Murphy, J., Spinelli, S., Gutierrez, C., Richet, N., Kellenberger, C., Desmyter, A., Mahony, J., van Sinderen, D., Cambillau, C.(2016) mBio 7: e01781-e01715
- PubMed: 26814179
- DOI: https://doi.org/10.1128/mBio.01781-15
- Primary Citation of Related Structures:
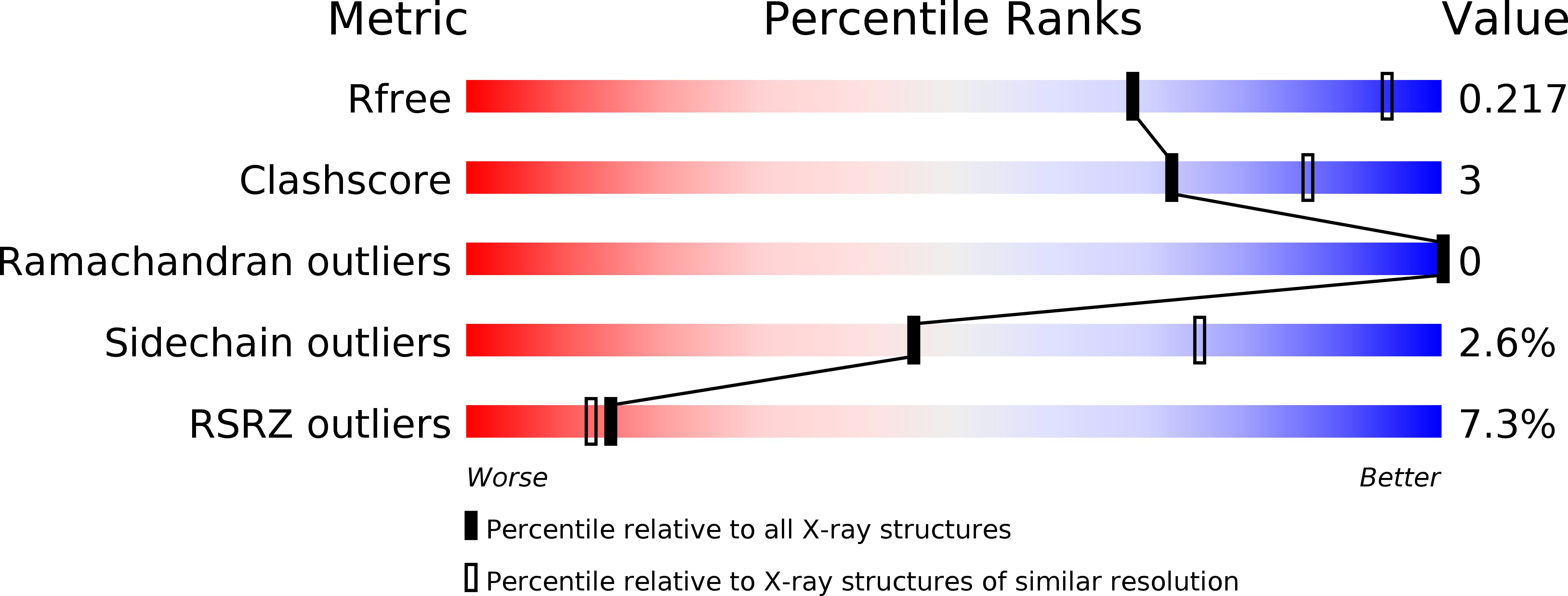
5E7B, 5E7F, 5E7T - PubMed Abstract:
The Gram-positive bacterium Lactococcus lactis, used for the production of cheeses and other fermented dairy products, falls victim frequently to fortuitous infection by tailed phages. The accompanying risk of dairy fermentation failures in industrial facilities has prompted in-depth investigations of these phages. Lactococcal phage Tuc2009 possesses extensive genomic homology to phage TP901-1. However, striking differences in the baseplate-encoding genes stimulated our interest in solving the structure of this host's adhesion device. We report here the X-ray structures of phage Tuc2009 receptor binding protein (RBP) and of a "tripod" assembly of three baseplate components, BppU, BppA, and BppL (the RBP). These structures made it possible to generate a realistic atomic model of the complete Tuc2009 baseplate that consists of an 84-protein complex: 18 BppU, 12 BppA, and 54 BppL proteins. The RBP head domain possesses a different fold than those of phages p2, TP901-1, and 1358, while the so-called "stem" and "neck" domains share structural features with their equivalents in phage TP901-1. The BppA module interacts strongly with the BppU N-terminal domain. Unlike other characterized lactococcal phages, Tuc2009 baseplate harbors two different carbohydrate recognition sites: one in the bona fide RBP head domain and the other in BppA. These findings represent a major step forward in deciphering the molecular mechanism by which Tuc2009 recognizes its saccharidic receptor(s) on its host. Understanding how siphophages infect Lactococcus lactis is of commercial importance as they cause milk fermentation failures in the dairy industry. In addition, such knowledge is crucial in a general sense in order to understand how viruses recognize their host through protein-glycan interactions. We report here the lactococcal phage Tuc2009 receptor binding protein (RBP) structure as well as that of its baseplate. The RBP head domain has a different fold than those of phages p2, TP901-1, and 1358, while the so-called "stem" and "neck" share the fold characteristics also found in the equivalent baseplate proteins of phage TP901-1. The baseplate structure contains, in contrast to other characterized lactococcal phages, two different carbohydrate binding modules that may bind different motifs of the host's surface polysaccharide.
Organizational Affiliation:
Synchrotron Soleil, L'Orme des Merisiers, Gif-sur-Yvette, France.