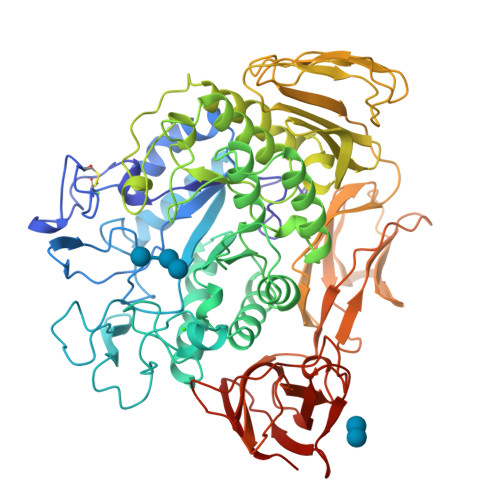
Substrate binding to a cyclodextrin glycosyltransferase and mutations increasing the gamma-cyclodextrin production.
Parsiegla, G., Schmidt, A.K., Schulz, G.E.(1998) Eur J Biochem 255: 710-717
- PubMed: 9738912
- DOI: https://doi.org/10.1046/j.1432-1327.1998.2550710.x
- Primary Citation of Related Structures:
4CGT, 5CGT, 6CGT, 7CGT, 8CGT, 9CGT - PubMed Abstract:
Bacterial cyclodextrin glycosyltransferases use starch to produce cyclic maltooligosaccharides (cyclodextrins) which are of interest in various applications. The cyclization reaction gives rise to a spectrum of ring sizes consisting of predominantly six to eight glucosyl units. Using the enzyme from Bacillus circulans strain no. 8, binding studies have been performed with several substrates and analogues. The observed binding modes differ in detail, but agree in general with data on homologous enzymes. Based on these binding studies, two mutations were designed that changed the production spectrum from the predominant product beta-cyclodextrin of the wild-type enzyme towards gamma-cyclodextrin, which is of practical interest because it is rare and can encapsulate larger nonpolar compounds.
Organizational Affiliation:
Institut für Organische Chemie und Biochemie, Freiburg im Breisgau, Germany.