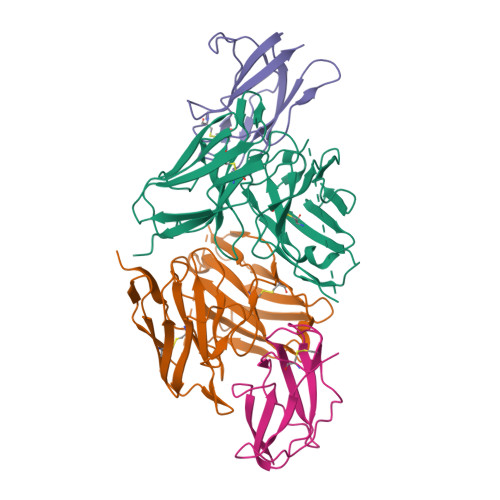
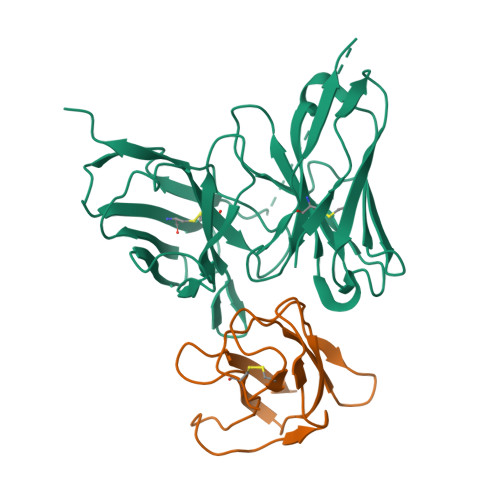
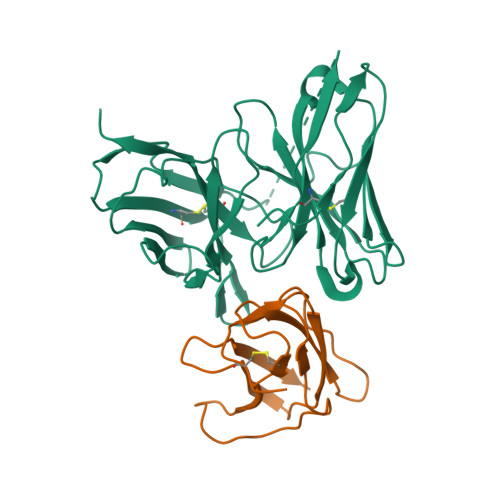
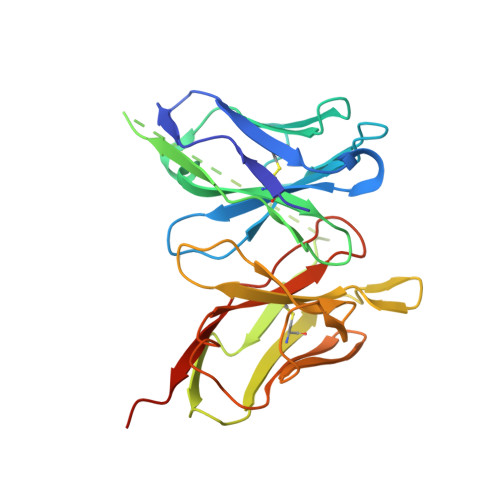
Structure-Guided Design of an Anti-Dengue Antibody Directed to a Non-Immunodominant Epitope.
Robinson, L.N., Tharakaraman, K., Rowley, K.J., Costa, V.V., Chan, K.R., Wong, Y.H., Ong, L.C., Tan, H.C., Koch, T., Cain, D., Kirloskar, R., Viswanathan, K., Liew, C.W., Tissire, H., Ramakrishnan, B., Myette, J.R., Babcock, G.J., Sasisekharan, V., Alonso, S., Chen, J., Lescar, J., Shriver, Z., Ooi, E.E., Sasisekharan, R.(2015) Cell 162: 493
- PubMed: 26189681
- DOI: https://doi.org/10.1016/j.cell.2015.06.057
- Primary Citation of Related Structures:
5AAM, 5AAW - PubMed Abstract:
Dengue is the most common vector-borne viral disease, causing nearly 400 million infections yearly. Currently there are no approved therapies. Antibody epitopes that elicit weak humoral responses may not be accessible by conventional B cell panning methods. To demonstrate an alternative strategy to generating a therapeutic antibody, we employed a non-immunodominant, but functionally relevant, epitope in domain III of the E protein, and engineered by structure-guided methods an antibody directed to it. The resulting antibody, Ab513, exhibits high-affinity binding to, and broadly neutralizes, multiple genotypes within all four serotypes. To assess therapeutic relevance of Ab513, activity against important human clinical features of dengue was investigated. Ab513 mitigates thrombocytopenia in a humanized mouse model, resolves vascular leakage, reduces viremia to nearly undetectable levels, and protects mice in a maternal transfer model of lethal antibody-mediated enhancement. The results demonstrate that Ab513 may reduce the public health burden from dengue.
Organizational Affiliation:
Visterra Inc., One Kendall Square, Suite B3301, Cambridge, MA 02139, USA; Department of Biological Engineering, Koch Institute of Integrative Cancer Research, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge MA 02139; USA.