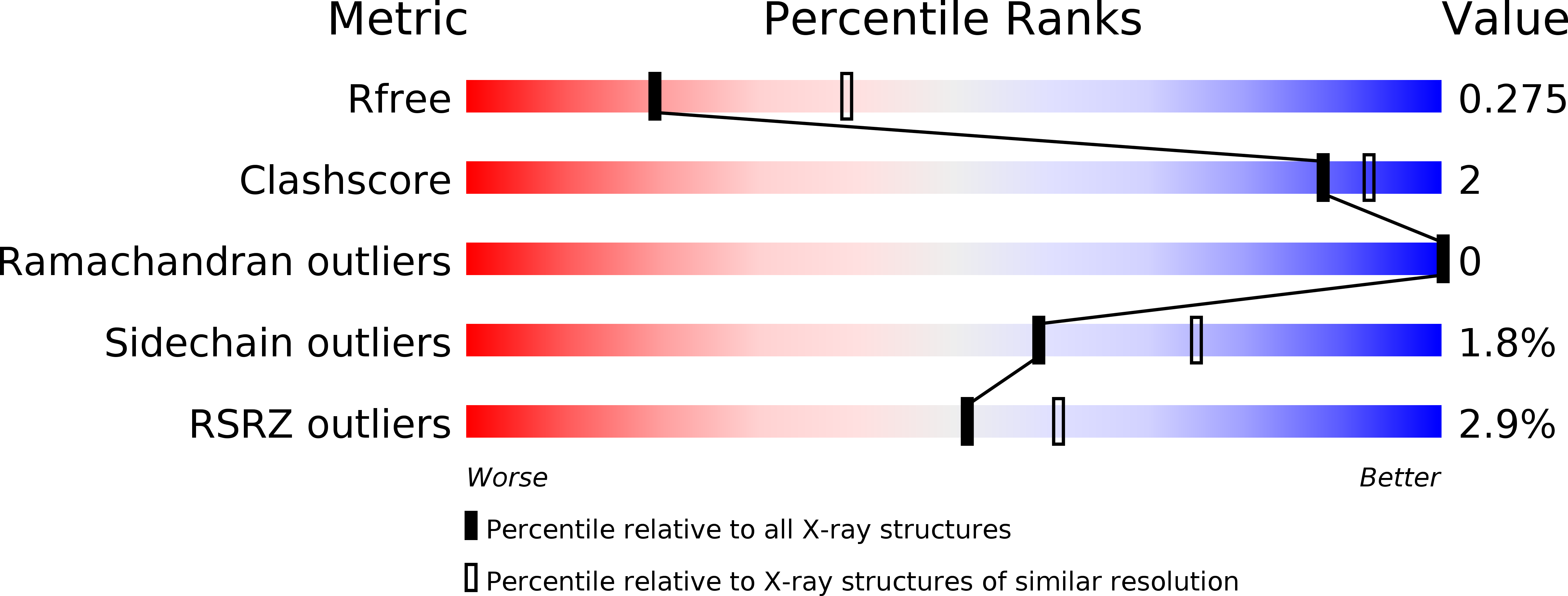
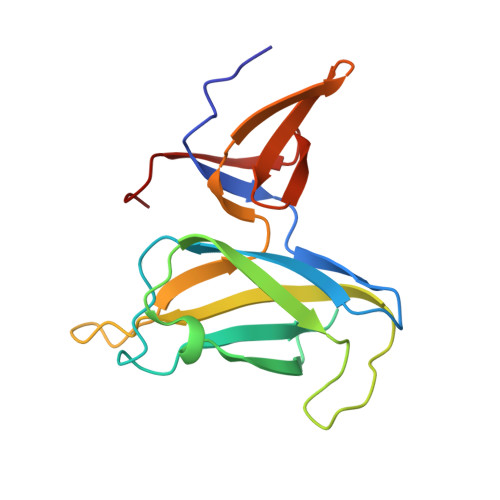
Crystal structure of truncated FlgD from the human pathogen Helicobacter pylori.
Pulic, I., Cendron, L., Salamina, M., Polverino de Laureto, P., Matkovic-Calogovic, D., Zanotti, G.(2016) J Struct Biol 194: 147-155
- PubMed: 26868107
- DOI: https://doi.org/10.1016/j.jsb.2016.02.003
- Primary Citation of Related Structures:
4ZZF, 4ZZK - PubMed Abstract:
Flagellin component D (FlgD) participates in the assembly of flagella, helical tubular structures that provide motility in non-filamentous bacteria. FlgD guides and controls the polymerization of FlgE that builds the hook, a short curved and hollow cylinder that connects the flagellar basal body spanning the cell envelope to the protruding filament. Crystal structures of truncated forms of Helicobacter pylori FlgD from two different strains in two space groups, I422 and P2, are reported here, at 2.2Å and 2.8Å resolution, respectively. Analogously to Pseudomonas aeruginosa and Xanthomonas campestris FlgD proteins, crystallization experiments set up for the full length protein resulted in crystals of a truncated form, lacking both N- and C-terminus ends. The crystal structures of the central domain show that the monomer is composed of a tudor and a fibronectin type III domain. The full length HpFlgD contains a long N-terminal signal region, probably partially flexible, a central globular region and a C-terminal segment with a peculiar repetitive pattern of amino acids. The spatial orientation of the two domains in HpFlgD differs from that of the homologous FlgD family members, P. aeruginosa and X. campestris. This difference together with the observation that HpFlgD assembles into tetramers, both in the solution and in the two crystal forms, strongly suggests that significant differences exist in the molecular organization of the flagella in different bacterial species.
Organizational Affiliation:
University of Zagreb, Faculty of Science, Department of Chemistry, Division of General and Inorganic Chemistry, Horvatovac 102a, Zagreb 10000, Croatia; Department of Biomedical Sciences, University of Padua, Via Ugo Bassi 58/B, Padua 35131, Italy.