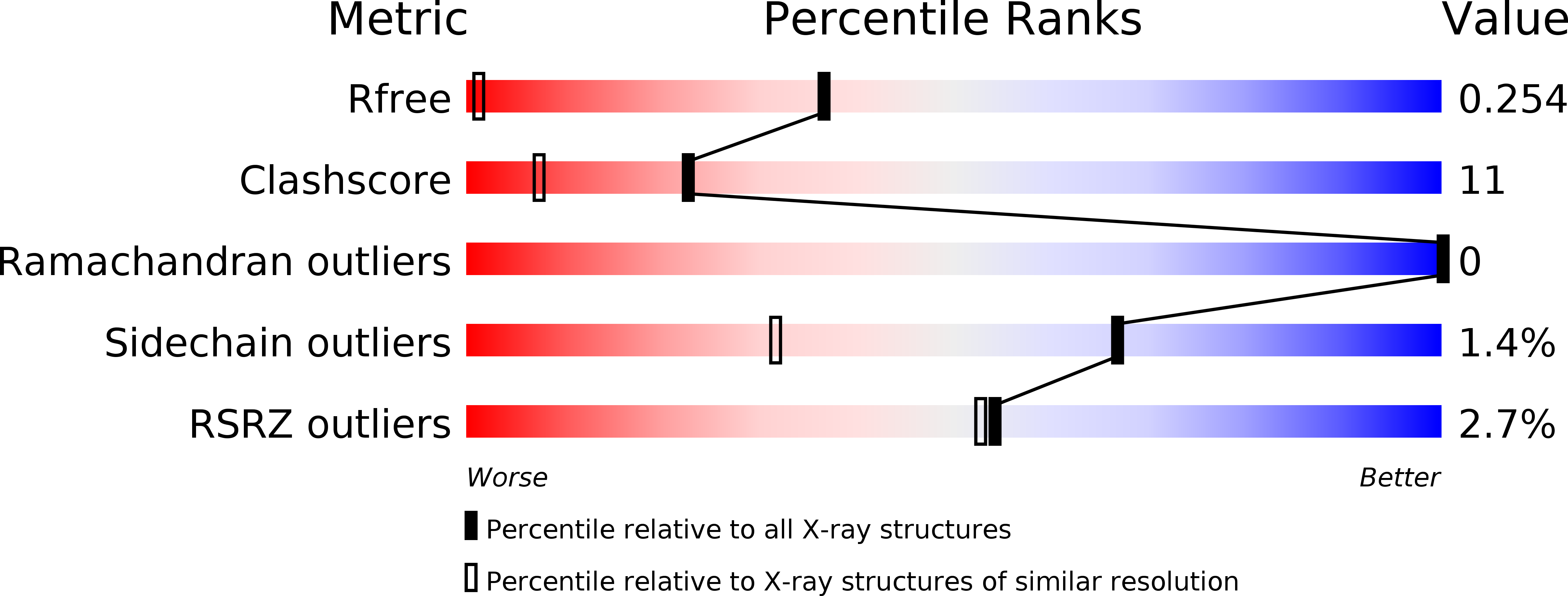
Mechanism of chitosan recognition by CBM32 carbohydrate-binding modules from a Paenibacillus sp. IK-5 chitosanase/glucanase
Shinya, S., Nishimura, S., Kitaoku, Y., Numata, T., Kimoto, H., Kusaoke, H., Ohnuma, T., Fukamizo, T.(2016) Biochem J 473: 1085-1095
- PubMed: 26936968
- DOI: https://doi.org/10.1042/BCJ20160045
- Primary Citation of Related Structures:
2RV9, 2RVA, 4ZXE, 4ZY9, 4ZZ5, 4ZZ8 - PubMed Abstract:
An antifungal chitosanase/glucanase isolated from the soil bacterium Paenibacillus sp. IK-5 has two CBM32 chitosan-binding modules (DD1 and DD2) linked in tandem at the C-terminus. In order to obtain insights into the mechanism of chitosan recognition, the structures of DD1 and DD2 were solved by NMR spectroscopy and crystallography. DD1 and DD2 both adopted a β-sandwich fold with several loops in solution as well as in crystals. On the basis of chemical shift perturbations in(1)H-(15)N-HSQC resonances, the chitosan tetramer (GlcN)4 was found to bind to the loop region extruded from the core β-sandwich of DD1 and DD2. The binding site defined by NMR in solution was consistent with the crystal structure of DD2 in complex with (GlcN)3, in which the bound (GlcN)3 stood upright on its non-reducing end at the binding site. Glu(14)of DD2 appeared to make an electrostatic interaction with the amino group of the non-reducing end GlcN, and Arg(31), Tyr(36)and Glu(61)formed several hydrogen bonds predominantly with the non-reducing end GlcN. No interaction was detected with the reducing end GlcN. Since Tyr(36)of DD2 is replaced by glutamic acid in DD1, the mutation of Tyr(36)to glutamic acid was conducted in DD2 (DD2-Y36E), and the reverse mutation was conducted in DD1 (DD1-E36Y). Ligand-binding experiments using the mutant proteins revealed that this substitution of the 36th amino acid differentiates the binding properties of DD1 and DD2, probably enhancing total affinity of the chitosanase/glucanase toward the fungal cell wall.
Organizational Affiliation:
Department of Advanced Bioscience, Kinki University, Nara 631-8505, Japan.