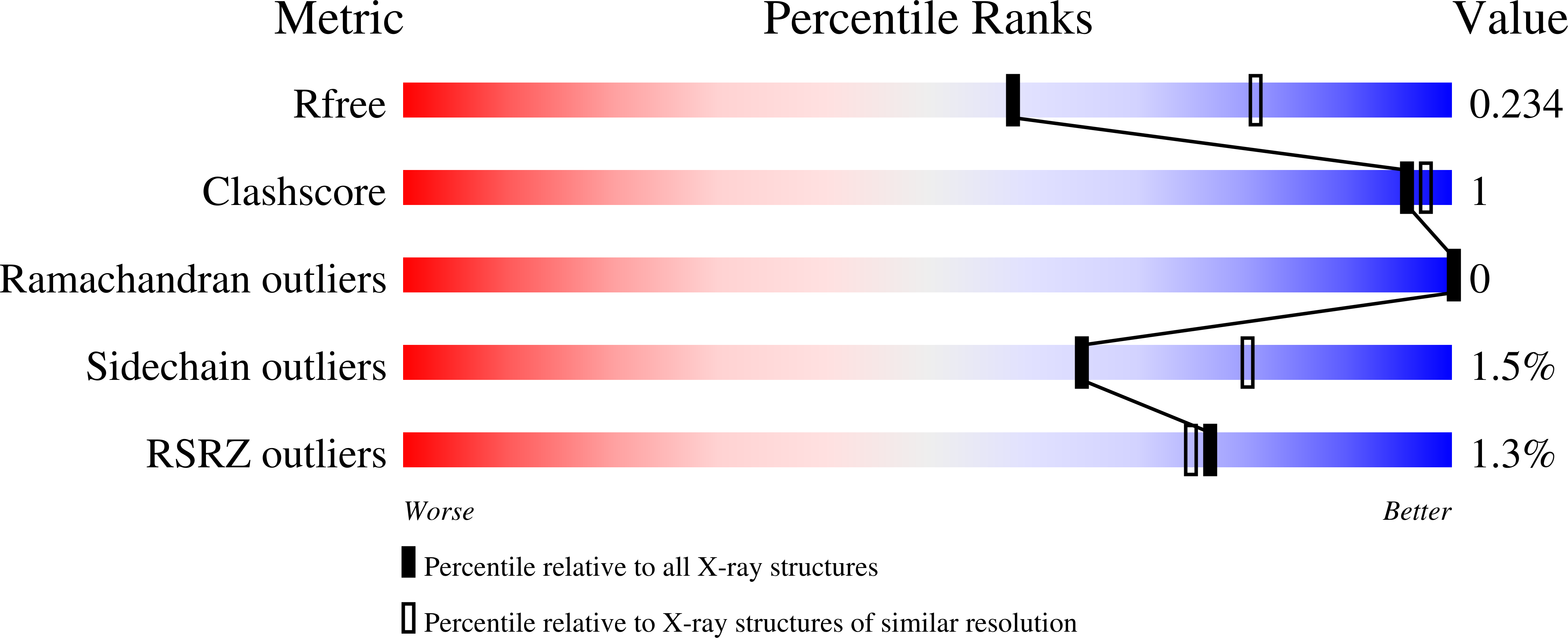
Structural basis for the enhancement of virulence by viral spindles and their in vivo crystallization.
Chiu, E., Hijnen, M., Bunker, R.D., Boudes, M., Rajendran, C., Aizel, K., Olieric, V., Schulze-Briese, C., Mitsuhashi, W., Young, V., Ward, V.K., Bergoin, M., Metcalf, P., Coulibaly, F.(2015) Proc Natl Acad Sci U S A 112: 3973-3978
- PubMed: 25787255
- DOI: https://doi.org/10.1073/pnas.1418798112
- Primary Citation of Related Structures:
4OW5, 4X27, 4X29, 4YN1, 4YN2 - PubMed Abstract:
The great benefits that chemical pesticides have brought to agriculture are partly offset by widespread environmental damage to nontarget species and threats to human health. Microbial bioinsecticides are considered safe and highly specific alternatives but generally lack potency. Spindles produced by insect poxviruses are crystals of the fusolin protein that considerably boost not only the virulence of these viruses but also, in cofeeding experiments, the insecticidal activity of unrelated pathogens. However, the mechanisms by which spindles assemble into ultra-stable crystals and enhance virulence are unknown. Here we describe the structure of viral spindles determined by X-ray microcrystallography from in vivo crystals purified from infected insects. We found that a C-terminal molecular arm of fusolin mediates the assembly of a globular domain, which has the hallmarks of lytic polysaccharide monooxygenases of chitinovorous bacteria. Explaining their unique stability, a 3D network of disulfide bonds between fusolin dimers covalently crosslinks the entire crystalline matrix of spindles. However, upon ingestion by a new host, removal of the molecular arm abolishes this stabilizing network leading to the dissolution of spindles. The released monooxygenase domain is then free to disrupt the chitin-rich peritrophic matrix that protects insects against oral infections. The mode of action revealed here may guide the design of potent spindles as synergetic additives to bioinsecticides.
Organizational Affiliation:
School of Biological Sciences, University of Auckland, Auckland, 1010, New Zealand;