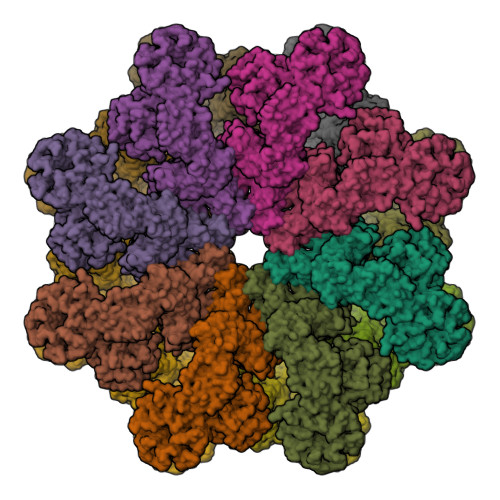
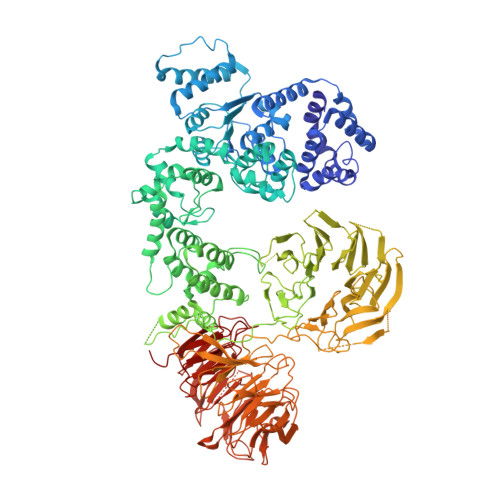
Structure of the Drosophila apoptosome at 6.9 angstrom resolution
Yuan, S., Yu, X., Topf, M., Dorstyn, L., Kumar, S., Ludtke, S.J., Akey, C.W.(2011) Structure 19: 128-140
- PubMed: 21220123
- DOI: https://doi.org/10.1016/j.str.2010.10.009
- Primary Citation of Related Structures:
4V4L - PubMed Abstract:
The Drosophila Apaf-1 related killer forms an apoptosome in the intrinsic cell death pathway. In this study we show that Dark forms a single ring when initiator procaspases are bound. This Dark-Dronc complex cleaves DrICE efficiently; hence, a single ring represents the Drosophila apoptosome. We then determined the 3D structure of a double ring at ∼6.9 Å resolution and created a model of the apoptosome. Subunit interactions in the Dark complex are similar to those in Apaf-1 and CED-4 apoptosomes, but there are significant differences. In particular, Dark has "lost" a loop in the nucleotide-binding pocket, which opens a path for possible dATP exchange in the apoptosome. In addition, caspase recruitment domains (CARDs) form a crown on the central hub of the Dark apoptosome. This CARD geometry suggests that conformational changes will be required to form active Dark-Dronc complexes. When taken together, these data provide insights into apoptosome structure, function, and evolution.
Organizational Affiliation:
Department of Physiology and Biophysics, Boston University School of Medicine, 700 Albany Street, Boston, MA 02118, USA.